Sporadic Hemangioblastoma in the Pituitary Stalk: A Case Report and Review of the Literature
Article information
Abstract
Hemangioblastomas in the pituitary stalk are especially rare. Most pituitary stalk hemangioblastomas reported in the literature were associated with von Hippel-Lindau (VHL) disease. Here, we report only the 3rd case of sporadic pituitary stalk hemangioblastoma diagnosed in a 60-year-old woman. Despite the danger of potential complications due to excessive vascularity or proximity to important neural structures, the tumor in our case was successfully removed while preserving pituitary function. In this case, complete surgical excision was shown to be an effective treatment option for symptomatic pituitary stalk hemangioblastoma, and we suggest careful evaluation of any highly enhancing mass with a signal void in the pituitary stalk preoperatively, even if no VHL disease is evident.
INTRODUCTION
Hemangioblastomas (HBLs) are World Health Organization (WHO) grade I capillary-rich neoplasms of the central nervous system (CNS) accounting for 1-2.5% of all intracranial neoplasms611). They can occur either sporadically (60-75% of cases) or in the context of von Hippel-Lindau (VHL) disease, the multi-organ neoplastic disorder that is inherited in an autosomal dominant manner (25-40% of cases)1112). The cerebellum is the most frequent location of these tumors; however, they may also occur in other regions of the CNS, medulla, and spinal cord11). Supratentorial HBLs are uncommon in about 100 cases reported in the literature614). HBLs in the pituitary stalk are extremely rare that only 14 cases have been reported to date : sporadic (2 cases); and combined with VHL disease (12 cases)256791011). We report a case of sporadic pituitary stalk hemangioblastoma with a satisfactory surgical resection. The differential diagnosis and management of these tumors is discussed with a review of the literature.
CASE REPORT
A 60-year-old female presented with a 2 month history of headache and dizziness. Her past medical history was unremarkable. Her family history was not significant and she had no stigmata of VHL disease. Pituitary function was normal. There was no neurologic deficit.
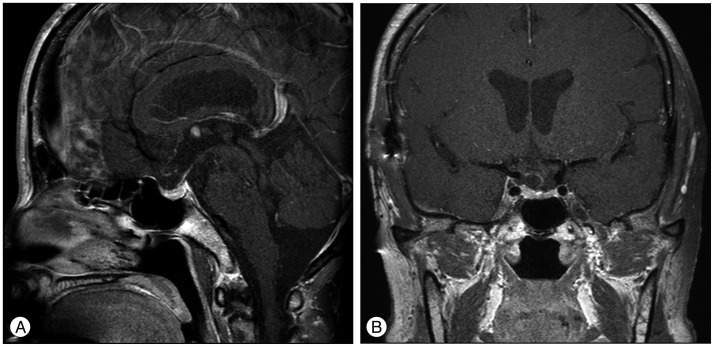
Brain magnetic resonance imaging (MRI) demonstrated an isointense suprasellar mass on the T1-weighted images. On the T2-weighted images, the mass was heterogenous in signal intensity. The mass exhibited marked homogenous contrast enhancement with intravenous administration of gadolinium and it appeared that the tumor was originated from the pituitary stalk. The mass was measured to be 10 to 12 mm with multiple signal voids inside, and no other intracranial lesion was identified (Fig. 1).
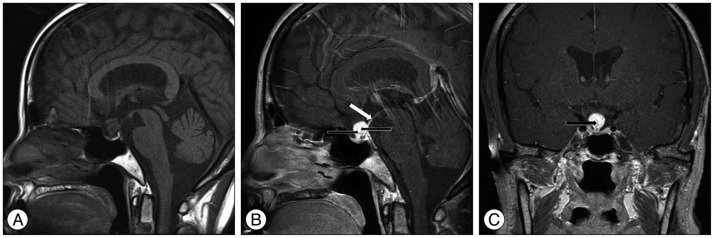
Preoperative sagittal T1-weighted magnetic resonance image without contrast enhancement reveals an isointense suprasellar mass 10 to 12 mm in dimension located on the pituitary stalk (A). Preoperative sagittal and coronal magnetic resonance images with contrast show strongly enhanced mass involving the pituitary stalk (white arrow) with multiple signal voids inside (black arrows) (B and C).
Operative resection of the mass in the suprasellar region was performed with a right frontotemporal craniotomy. In the surgical fields, an obviously hyperemic and friable round mass with a thin capsule located mainly in the ventrocaudal portion of the pituitary stalk was identified. It was adherent to the paper thin leafs of the pituitary stalk. Adhesion to the diaphragma sellae was not observed. During the operative resection, brisk bleeding due to the vascularity of the lesion was controlled with a bipolar coagulator and hemoclip. The mass that contained the lump of blood vessels was gross totally extirpated (Fig. 2).

Intraoperative photograph shows a hyperemic, round mass with a thin capsule (white arrow) (A). The paper thin leafs of the pituitary stalk (asterisk) is revealed after total removal of tumor mass (B).
Histological findings showed a vascularized mass composed of numerous thin-walled vascular channels lined endothelial cells and numerous ovoid, vacuolated stromal cells in a hematoxyline and eosin stain. This is consistent with the typical well-known morphology of HBLs. Mitotic figures were rare. Immunohistochemical staining was performed with antibody for CD 34. Endothelial cells showed a positive reaction for CD 34 (Fig. 3).

Photomicrographic view of the tumor specimens showing a vascular lesion composed of prominent thin-walled vessels with vacuolated stromal cells (Hematoxylin and eosin, ×50) (A). Blue circles indicate positively stained endothelial cells (Immunostain for CD34 stain, ×200) (B).
Postoperatively, the patient experienced considerable improvement of her symptoms and the postoperative period was uneventful except for occurrence of transient diabetes insipidus which was successfully managed with fluid control and hormonal replacement therapy. During the follow up period, a manifestation of the VHL complex was investigated and screening for other HBLs was performed, but there were no remarkable findings. Cranial MR images, obtained 6 months after surgery, demonstrated an intact pituitary stalk and no residual tumor (Fig. 4). Up to the present, 1 year after surgery, she lives well without neurological deficits or pituitary dysfunction.
DISCUSSION
HBLs are benign, highly vascular neoplasms of uncertain histogenesis10). While HBLs are frequently located in the posterior fossa, they may rarely be originated from their supratentorial counterpart5614). They occur sporadically or as a manifestation of VHL disease with an autosomal dominant inheritance1112). HBLs in the CNS are the most common clinical features of VHL disease and are often multiple. Generally, HBLs are distributed in a highly conserved, region-specific manner within the CNS that includes the retina, brainstem, spinal cord, and cerebellum regardless of genetic predisposition10). Therefore, supratentorial HBLs including pituitary stalk HBLs are rare, accounting for only 2.1-2.9% of all HBLs181114). Furthermore, HBLs occurring in the pituitary stalk are extremely rare with only 14 reported cases256791011).
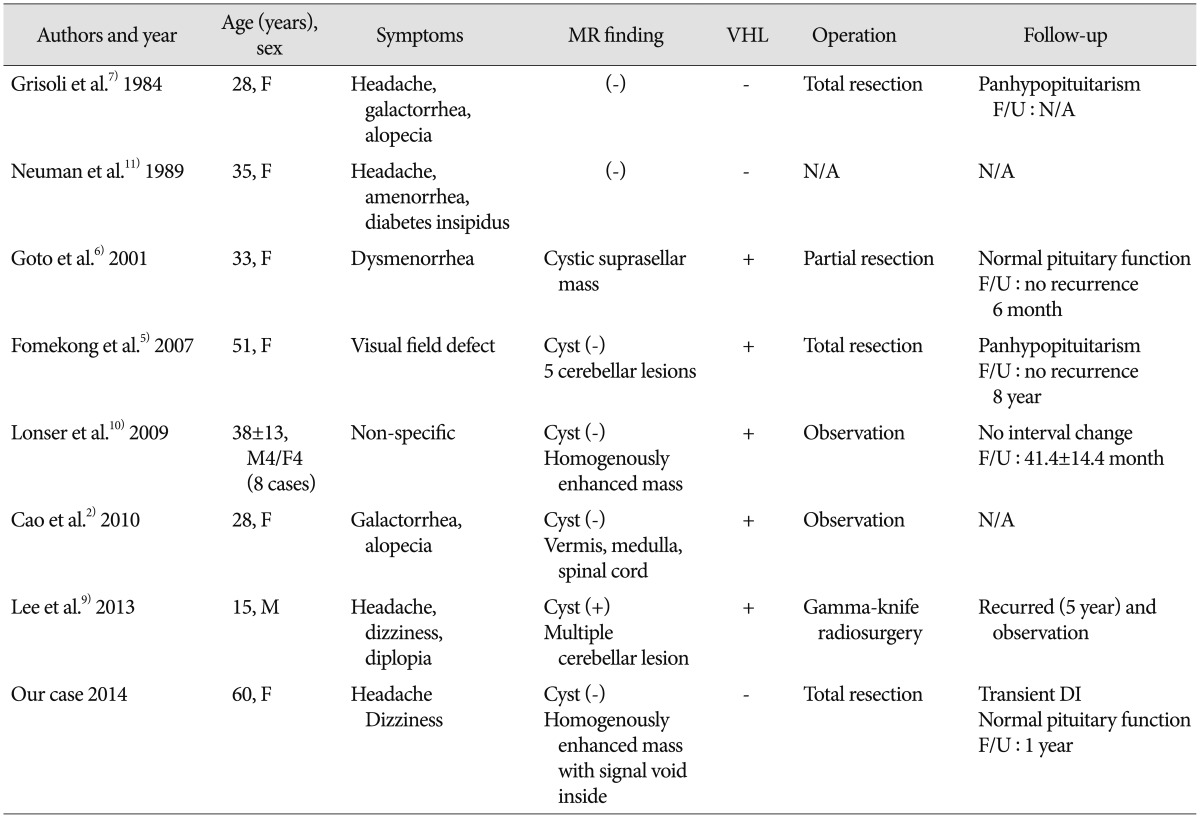
We summarized the features of pituitary stalk HBLs along with the present case in Table 1. In 14 cases of pituitary stalk HBL (9 females and 5 males) previously described in the literature, the mean age of the patients was 32.6 years (range, 11-74 years). Headache, endocrinological abnormalities, and visual disturbance were the leading causes of hospital visitation; however, 8 patients with VHL diseases described by Lonser et al.10) were asymptomatic. Of the 14 patients, 12 patients were associated with VHL disease. The predominant type of HBLs in the CNS is sporadic HBL; however those in the pituitary stalk have a countertrend even if there are small numbers of cases.
Although two patients with sporadic pituitary stalk HBL were evaluated with computed tomography and conventional angiography, MR imaging was performed in all the previously reported 12 cases of HBLs with VHL disease. The tumors were consistently isointense on T1- and hyperintense or heterogenous on T2 weighted images with marked enhancement after intravenous injection of contrast material. We were able to show the same radiologic findings in our sporadic case with an additional signal void within the tumor. The signal void on the T2 weighted image or the T1 enhancement image usually demonstrates intratumoral vascular structures like feeding vessels or a cluster of blood vessels13). In addition, the mass was beyond the diaphragma sellae and absent a dural "tail" sign that is a characteristic finding of meningioma. These findings enable us to discriminate pituitary stalk HBLs and other tumorous conditions arising from the suprasellar region. As stated above, most of the pituitary stalk HBLs were associated with VHL disease (12 of 14 cases). Accordingly, a pituitary stalk mass in a VHL patient can be highly suggested as an HBL, and it is necessary to rule out VHL disease in patients with an isolated pituitary stalk mass that is similar to HBLs. Furthermore, although an accurate preoperative diagnosis for these lesions would be very challenging in patients with neither other lesions nor VHL disease, a sporadic and isolated pituitary stalk HBL needs to be considered when an MR imaging study reveals the characteristic findings as shown in the present study.
Surgical resection of pituitary stalk HBLs was reported in three cases and panhypopituitarism was developed in 2 patients who underwent grossly total resection567). Although resection was complete in our case, no pituitary dysfunction was noted except transient diabetes insipidus. Lonser et al.10) suggested that asymptomatic pituitary stalk HBLs do not need to undergo tumor resection because of potential surgery-related morbidity and the slow growth pattern. Moreover, the excessive vascularity of the lesion and its proximity to critical neural structures including the pituitary stalk, optic apparatus, and hypothalamus sometimes precludes complete excision14). However, we thought that complete surgical excision was the optimal treatment strategy for sporadic pituitary stalk HBLs when its rising growth pattern or symptomatic presentation is taken into account9). This is because subtotal excision is associated with a high incidence of local disease progression requiring consideration of adjuvant radiosurgery36). Although the role of radiation therapy in the treatment of HBLs remains controversial, primary or adjuvant radiotherapy, either fractionated or single-dose such as gamma-knife radiosurgery, can be useful to treat HBLs14). In this case, even gentle dissection with a micro-dissector caused bleeding from the lesion and only after small vessels that were found embedded posteriorly to the mass were coagulated with a bipolar coagulator and hemoclip, the bleeding was controlled and an operative field was clearly visualized.
Histological differential diagnosis of HBLs was made with hemangiopericytoma. It also may be difficult to differentiate between HBLs, renal cell carcinoma, and capillary angioma45). HBLs have heterogeneity in a ratio of vasculature to the tumor cells. Frequently, highly vascular thin-walled vessels surrounded by vacuolated stromal cells were observed. Mitotic figures are rare. Immunohistologically, HBLs have unique characteristics in staining positive for CD34 only in endothelial cells and negative in stromal cells as in our case4).
CONCLUSION
We present only the 3rd case of a sporadic pituitary stalk HBL. Despite the danger of potential complications due to excessive vascularity or proximity to important neural structures, the tumor in our case was successfully removed without a significant complication. In this case, complete surgical excision was shown to be a feasible treatment option for symptomatic pituitary stalk hemangioblastoma. Therefore, we suggest careful evaluation of any highly enhancing mass with a signal void in the pituitary stalk preoperatively, even if no VHL disease is evident.