Is There Additive Therapeutic Effect When GCSF Combined with Adipose-Derived Stem Cell in a Rat Model of Acute Spinal Cord Injury?
Article information
Abstract
Objective
Functional and neural tissue recovery has been reported in many animal studies conducted with stem cells. However, the combined effect of cytokines and stem cells has not yet been adequately researched. Here, we analyzed the additive effects of granulocyte colony-stimulating factor (GCSF) on adipose-derived stem cells (ADSCs) infusion in the treatment of acute spinal cord injury (SCI) in rats.
Methods
Four days after intrathecal infusion tubes implantation in Sprague-Dawley rats, SCI was induced with an infinite horizon impactor. In the Sham group (n=5), phosphate-buffered saline was injected 3, 7, and 14 days after SCI. GCSF, ADSCs, and ADSCs with GCSF were injected at the same time in the GCSF (n=8), ADSC (n=8), and ADSC+GCSF groups (n=7), respectively.
Results
The ADSC and ADSC+GCSF groups, but not the GCSF group, showed significantly higher Basso-Beattie-Bresnahan scores than the Sham group during 8 weeks (p<0.01), but no significant difference between the ADSC and ADSC+GCSF groups. In the ladder rung test, all four groups were significantly different from each other, with the ADSC+GCSF group showing the best improvement (p<0.01). On immunofluorescent staining (GAP43, MAP2), western blotting (GAP43), and reverse transcription polymerase chain reaction (GAP43, nerve growth factor), the ADSC and ADSC+GCSF groups showed higher levels than the Sham and GCSF groups.
Conclusion
Our analyses suggest that the combination of GCSF and ADSCs infusions in acute SCI in the rat does not have a significant additive effect. Hence, when combination agents for SCI stem cell therapy are considered, molecules other than GCSF, or modifications to the methodology, should be investigated.
INTRODUCTION
Spinal cord injury (SCI) in humans leads to permanent functional deficits in the adult central nervous system3). Functional recovery can be achieved by endogenous repair mechanisms such as endogenous stem cell proliferation30,45), remyelination40), and neural plasticity2). However, severe SCI can cause the permanent neurological deficit. These deficits include neural cell death and axonal degeneration in the lesioned tissue45). Clinical treatment of SCI is focused on controlling the secondary damage after the occurrence of the lesion in recent days30). However, controlling the secondary damage in the injured spinal column is not necessarily accompanied by restoration of spinal function40). Therefore, repair strategies for SCI require the production of an amicable environment within the injured spinal cord in order to protect injured neurons from the effects of secondary damage and also to facilitate axonal regeneration24). In recent years, many studies on the use of stem cells in animal models of SCI have been performed and both functional and neural tissue recovery have been reported2,6,10,21,24,36).
Adult stem cells are unspecialized cells capable of long-term self-renewal and differentiation into specialized cells. They can thus be transformed into the various types of cells that comprise diverse body organs, including bones, brain, muscles, and skin26). Adult stem cells are able to modify the pro-inflammatory response, reducing inhibitory effects of glial scar and promoting a permissive environment for axonal elongation43,49). Furthermore, undifferentiated mesenchymal stem cells (MSCs) have been applied to a clinical trial with these expected mechanisms for SCI30). Adipose tissue is a rich source of pluripotent stem cells that are similar to the MSCs from bone marrow16). Adipose-derived stem cells (ADSCs) are more useful because they are abundant and easily extractable13). These ADSCs have shown therapeutic effects in various studies7,17,35,37,38). Granulocyte colony-stimulating factor (GCSF) was developed and commercialized as an anticancer drug4). Nowadays, however, it is also reported to be a neurotrophic factor that affects the hematopoietic system, induces the formation of the nerves of the central nervous system, increases synaptic plasticity, and mobilize stem cells from bone marrow9,47). In addition, several studies reported on the inhibitory effect of GCSF on neuronal cell death in the brain and spinal cord11,18,47).
ADSC and GCSF were reported to have a therapeutic effect in SCI independently, but the combined use of ADSCs and GCSF are not promising currently because this has not been well studied yet. Therefore, if we find a synergistic effect when using them together, it will provide clinical significance. Thus, in our present study, we hypothesized that GCSF could exert synergic effect especially by functioning as neurotrophic factor combined with the neurotrophic effect of ADSC and reducing secondary damage after SCI and analyzed with the behavioral test, immunofluorescent staining, and molecular study in a rat model of SCI.
MATERIALS AND METHODS
Animals
This study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) Asan Institute for Life Sciences, Asan Medical Center. The committee abides by the institute of Laboratory Animal Resources (ILAR) guide (2012-02-029). Female Sprague-Dawley (SD; Orient Bio, Seongnam, Korea) rats weighing 250–280 g at the time of injury were used. Before the injury, all rats were housed at two animals per cage under simulated daylight conditions with alternating 12 hours light-dark cycles and had free access to food and water.
Spinal cord injury
We inserted the intrathecal (IT) catheters prior to SCI to confirm there was no spinal cord injury by IT catheter insertion. Four days before SCI induction, all rats underwent implantation of polyethylene tube (PE10; Becton Dickinson, Rutherford, NJ, USA) with outer diameter 0.61 mm (inner diameter: 0.28 mm) to receive IT stem cell infusion. Prior to implant surgery, rats were administered with inhalation anesthesia using 2% isoflurane (Hana Pharm, Hwaseong, Korea). The IT catheters were inserted through cisterna magna to avoid spinal cord damage and pushed 30 mm downwardly. After tube implantation, the external tube tip was closed and sterilized with an alcohol lamp and remained embedded in the subcutaneous layer. We applied IT catheters to all groups including Sham group to avoid the interferences caused by the catheter surgery. Among the rats, motor deficits occurred immediately after IT catheter insertion in two rats, which were excluded in this experiment. After four days of observation period on the wound healing and recovery from the stress of catheter insertion, the vertebral column was exposed between T7 and T8 under the same inhalation anesthesia, and a bilateral dorsal laminectomy was performed at T7 without rupturing the dura. A spinal cord contusion injury was induced by a 200 kdyn force through the use of an infinite horizon (IH) impactor (Precision System and Instrumentation, Fairfax, VA, USA). After SCI induction, the skin was primarily closed, and rats were given a prophylactic subcutaneous injection of cefazolin (10 mg/kg). For pain management, all animals were subcutaneously injected with ketoprofen (5 mg/kg) once daily for three days. After injury induction, the rats were housed individually in the same pre-injury environment. Bladders were emptied manually twice a day until spontaneous control of urination returned.
Cell preparation
All cell lines were provided by Prostemics (Seoul, Korea). 10 g of fat tissue was harvested from the bilateral inguinal area of the rat. Harvested fat tissue was washed in saline and digested in 0.075% collagenase type II (Sigma-Aldrich, St. Louis, MO, USA; filtered with a 0.20 μm syringe filter) at 37°C for 1 hour. The harvested fat tissue was treated with an equal volume of low-glucose Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Rockville, MD, USA) containing 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco). Cells were collected by centrifugation for 10 min at 600×g, and the supernatant was discarded. The pellet was re-suspended in 160 mM ammonium chloride (Sigma-Aldrich) and incubated for 3 min at room temperature. ADSCs were isolated by mash filtration (70 μm nylon mesh filter) and centrifuged at 600×g for 10 minutes. The pellet was suspended in a 75 cm2 flask (Nunc, Rochester, NY, USA) as the primary culture and incubated at 37°C under 5% CO2. Non-adherent cells were removed by replacing the medium. Cells were fed every 3 days with 10 mL of complete DMEM media. When this primary culture of ADSCs reached 80% confluence, cells were harvested using 0.25% trypsin-EDTA and washed in 10 mL of phosphate-buffered saline (PBS). Cell viability was assessed by Trypan blue staining.
ADSCs of passage 3 cultured in a control medium for 48 hours prior to analysis were incubated with monoclonal PE-conjugated antibodies for CD34 and CD45 (BD Pharmingen, San Diego, CA, USA) or with FITC conjugated antibodies for CD73 and CD105 (BD Pharmingen) for 30 minutes at room temperature. As a control, cells were stained with isotype control IgG. All the cells stained with antibodies were subsequently washed with PBS, fixed with 4% formaldehyde, and analyzed on a FACScan flow cytometer (Beckton Dickson, San Jose, CA, USA) using CELL Quest Pro software.
GCSF injection and cell transplantation
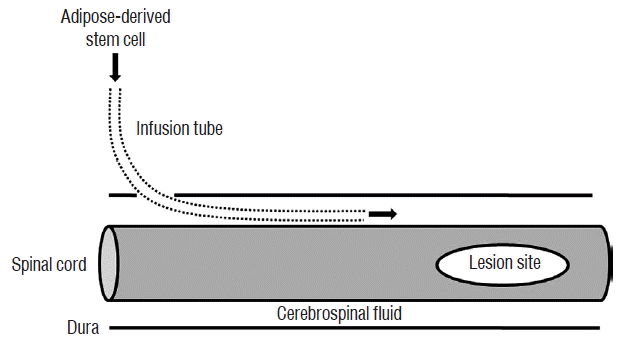
Three days after the induction of cord injury, the rats were divided into four groups. Human recombinant GCSF (5 μg/100 g; Dong-A, Seoul, Korea) was injected via the tail vein in the GCSF group (n=8). ADSCs (1×107 cells) in 50 μL of PBS were infused via the IT tube in the ADSC group (n=8), and IT ADSCs infusion with intravenous GCSF injection was performed in the ADSC+GCSF group (n=7). In the Sham group (n=5), PBS (50 μL) was injected via the IT tube. GCSF was injected in GCSF and ADSC+GCSF groups at 3, 7, and 14 days after SCI. ADSCs were infused in ADSC and ADSC+GCSF groups at 3, 7, and 14 days after SCI. During each cell infusion, the animals were anesthetized and the external tip of the IT tube was re-exposed (Fig. 1). The ADSCs were infused at a rate of 10 μL per minute. After ADSCs infusion, the external tube tip was closed and sterilized with an alcohol lamp. The surgical site was then re-closed.
Behavioral testing
Open field testing procedures for Basso-Beattie-Bresnahan (BBB) locomotor rating scale were performed for all rat groups as previously described1,3,8,43). Before these tests, each animal was allowed to adapt to a treadmill (0.15 m/s). Once a rat demonstrated acclimatization by walking continuously on the treadmill, locomotor performance was recorded with a video camera for about 5 minutes. An examiner who was blind to any group-identifying information used the video to quantitatively assess the locomotor performance of each rat according to the BBB scale. Tests were performed on the 1st, 2nd, and 3rd day after the injury, and then weekly for 8 weeks. Ladder rung tests were performed on a 1.2 m runway. A total of 80 rungs (bars) of 0.5 cm in diameter and separated by 1.5 cm were installed. The number of adequate steps for every ten steps on the bars was and the evaluation was repeated three times. Tests were performed weekly for 8 weeks after SCI.
Immunofluorescent study
Eight weeks after SCI, all rats were sacrificed and perfused with PBS and 4% paraformaldehyde (PFA; Sigma-Aldrich). Twenty millimeters length of spinal cords centered on the lesion site were extracted and fixed with 4% PFA for 6 hours followed by incubation with 30% sucrose in PBS overnight. Serial longitudinal sections with 10 μm thickness of the spinal cord were obtained in every 50 μm with a cryotome (Microm, Walldorf, Germany) and mounted on poly-L-lysine-coated Superfrost Plus slides (Matsunami, Osaka, Japan). The sections were washed three times with Tris-buffered saline (TBS), then blocked with TBS containing 5% bovine serum albumin and 0.1% Triton X-100 for 1 hour at room temperature. The sections were incubated overnight with primary antibody at 4°C, washed three times with TBS, and then incubated with the fluorescent-conjugated secondary antibody (1: 1000; Invitrogen, Carlsbad, CA, USA). As primary antibodies, monoclonal antibodies against growth associated protein 43 (GAP43, 1: 200; Abcam, Cambridge, UK), microtubule-associated protein 2 (MAP2, 1: 200; Abcam), and choline acetyltransferase (ChAT, 1: 200; Abcam) were used. Sections were observed under a fluorescence microscope (Carl Zeiss Meditec, Jena, Germany).
Western blotting
Injury epicenters of 0.5 cm in size, harvested 8 weeks after SCI, were lysed by homogenization in 300 μL lysis buffer containing 20 mM Tris pH 7.4, 50 mM NaCl2, 1% Triton X-100, and protease inhibitor (Intron, Seoul, Korea). Debris was cleared by centrifugation, and supernatants were aliquoted and stored at −80°C. Samples were assayed for protein concentration with a bicinchoninic acid assay (Thermo Scientific, Rockford, IL, USA). After heating at 100°C for 5 minutes in 5 × sample buffer, equal amounts of denatured protein (30 μg) were separated by SDS-polyacrylamide gel electrophoresis on 10% gels and transferred onto polyvinylidene difluoride membranes at 20 V for 7 minutes with an iBlot® gel transfer device (Invitrogen). After incubating for 1 hour at room temperature with blocking solution (0.4% Tween-20 and 5% dry milk in PBS), membranes were incubated overnight with primary antibodies against serotonin transporter (1: 1000; Abcam), acetylcholine receptor (1: 1000; Abcam), or GAP43 (1: 500; Abcam); an antibody against β-actin (1: 10000; Sigma-Aldrich) was used as a control for equal loading. After washing with 0.1% Tween-20/TBS, blots were incubated with horseradish peroxidase-conjugated secondary antibodies (Sigma-Aldrich) and then developed with enhanced chemiluminescence reagents (Pierce Biotechnology, Rockford, IL, USA).
Reverse transcription polymerase chain reaction (RT-PCR)
For RNA isolation, 1 mL TRIzol® reagent (Invitrogen) was added per 100 mg of spinal cord specimen, homogenized with a pestle and mortar and liquid nitrogen, and washed with 1 mL diethylpyrocarbonate-treated water (Invitrogen). Homogenized samples were incubated for 5 minutes at room temperature and 200 μL of chloroform (Sigma-Aldrich) was added per 1 mL of TRIzol® reagent. The samples were vigorously hand shaken for 15 seconds, incubated for 3 minutes, and divided following centrifugation at 12000×g for 10 minutes at 4°C. The aqueous phase was transferred to a fresh tube (1.5 mL) and 500 μL of isopropyl alcohol (Sigma-Aldrich) was added per 1 mL of TRIzol® reagent. The samples were incubated at room temperature for 10 minutes and centrifuged at 12000×g for 10 minutes at 4°C. The RNA was washed by centrifugation at 7500×g for 5 minutes at 4°C after supernatant removal and 1 mL of 75% ethanol was added per 1 mL of TRIzol® reagent. The RNA pellet was briefly dried and the RNA was dissolved in RNase-free water and incubated for 10 minutes at 60°C. The cDNA mixture was incubated at 65°C for 5 minutes, placed on ice for 1 minute, and then incubated at 50°C for 50 minutes in the following cDNA synthesis mixture: 2 μL 10×reverse transcriptase buffer, 4 μL 25 mM MgCl2, 2 μL 0.1 M DTT, 1 μL RNase OUT™ (40 U/μL; Invitrogen), and 1 μL Superscript™ III RT (200 U/μL; Invitrogen). The mixture was placed at 85°C for 5 minutes, chilled on ice, and then briefly centrifuged. Following the addition of 1 μL of RNase H, the sample was incubated for 20 minutes at 37°C. For the PCR mixture, sense and antisense primers (Table 1), template DNA, and distilled water were used. After the PCR reaction, correct band amplification was confirmed by agarose electrophoresis.
Data processing and statistical analyses
The results of behavior scores and molecular biological test in all four groups were analyzed by one-way analysis of variance followed by a posthoc Tukey-Kramer multiple comparison tests. Data are expressed as the mean±standard deviation. All statistical analyses were performed with SPSS statistical software (version 12.0.1; SPSS Inc., Chicago, IL, USA).
RESULTS
Characterization of ADSCs
ADSCs expanded easily in vitro by culturing and exhibited a fibroblast-like morphology. In flow cytometry, characteristic expressions of stem cell related surface markers were confirmed. ADSCs expressed CD73 and CD105 which are adipogenic mesenchymal stem cell markers in 90.1% and 98.6%, respectively and were negative in CD34 and CD45 representing hematopoietic stem cell markers in 96.2% and 99.4%, respectively (Fig. 2)38).
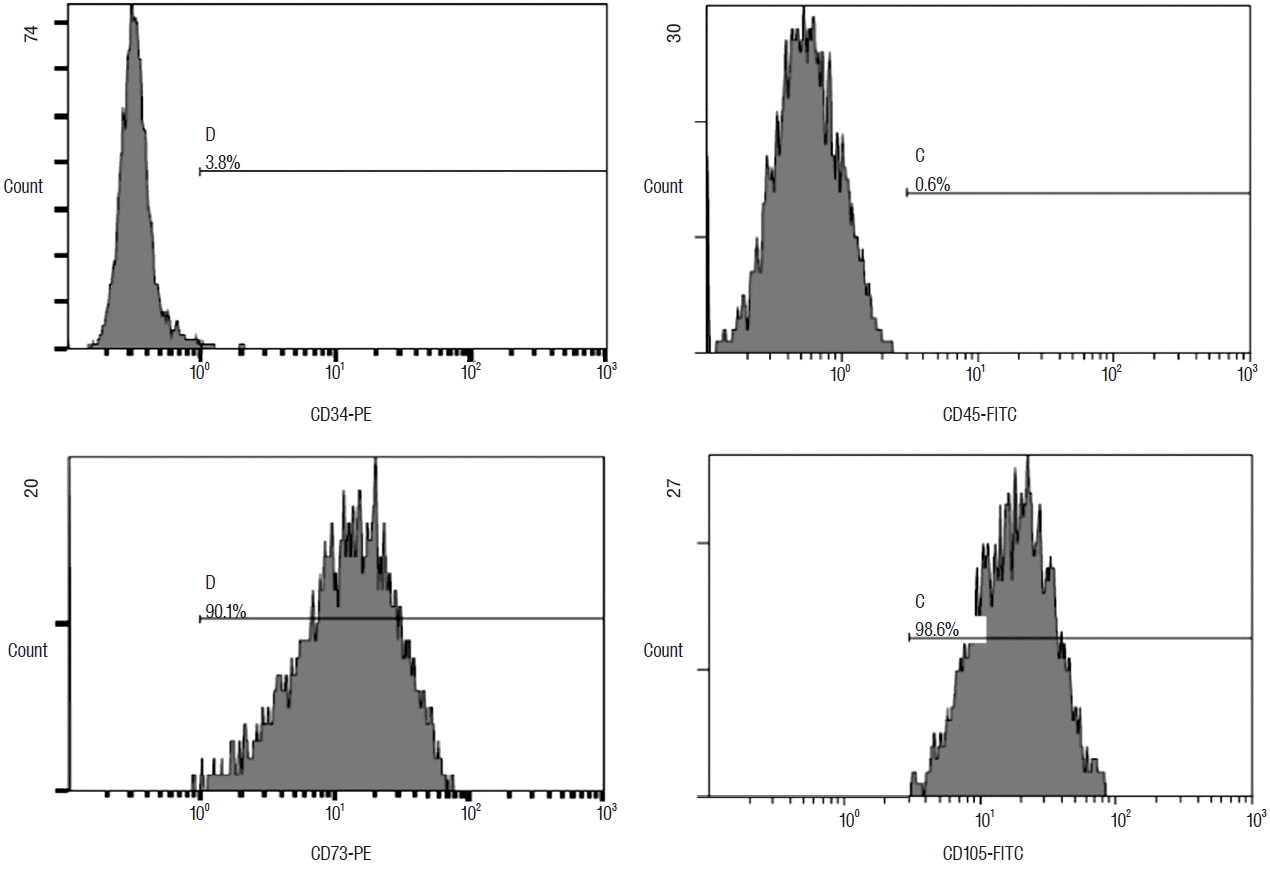
Flow cytometric histograms of rat ADSCs by FACScan flow cytometer (Beckton Dickson, San Jose, CA, USA). Adipogenic positive marker, CD73, and CD105 were expressed in 90.1% and 98.6%, respectively. Adipogenic negative marker, CD34, and CD45 were indicated as 3.8% and 0.6%, respectively. ADSC: adipose-derived stem cell.
Behavior tests
Eight weeks after SCI, rats in the ADSC and ADSC+GCSF groups achieved mean BBB scores of 17.37±0.7 and 17.57±0.5, respectively, which were significantly higher than the GCSF and Sham group BBB scores of 16±0.5 and 15.6±0.5, respectively (p<0.01; Fig. 3). There was no significant difference found between the BBB scores of the ADSC and ADSC+GCSF groups. The rats of the GCSF group did not show significantly higher score than that of the Sham group. In the ladder rung test, rats in the ADSC+GCSF group achieved a success rate of 70±2.8%, which was significantly higher than that of the Sham (52±3.2%), GCSF (62.5±3.7%), and ADSC (66.2±4.6%) groups (p<0.01; Fig. 4).

BBB locomotor scale test. The recovery of BBB locomotor function after SCI in rats treated by infusion with PBS (Sham group), injection with GCSF (GCSF group), infusion with ADSCs (ADSC group), or infusion with ADSCs and GCSF (ADSC+GCSF group). Black arrow is injection time point. *p<0.01. BBB: Basso-Beattie-Bresnahan, SCI: spinal cord injury, PBS: phosphate-buffered saline, GCSF: granulocyte colony-stimulating factor, ADSC: adipose-derived stem cell.
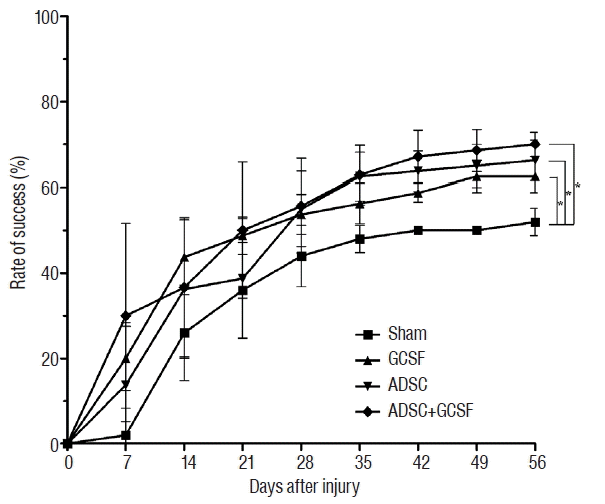
Ladder rung test. The recovery of hindlimb function after SCI in rats treated by infusion with PBS (Sham group), injection with GCSF (GCSF group), infusion with ADSCs (ADSC group), or infusion with ADSCs and GCSF (ADSC+GCSF group). *p<0.01. SCI: spinal cord injury, PBS: phosphate-buffered saline, GCSF: granulocyte colony-stimulating factor, ADSC: adipose-derived stem cell.
Immunofluorescent study
Confocal microscope analysis showed that GAP43 immunoreactivity in the injured spinal cords of the rats was higher in the ADSC and ADSC+GCSF groups than in the Sham and GCSF groups at 8 weeks after SCI (Fig. 5). For the other axonal regeneration marker, MAP2, microscope analysis showed higher MAP2 immunoreactivity in the injured spinal cord in the ADSC and ADSC+GCSF groups than in the Sham and GCSF groups 8 weeks after SCI (Fig. 6). There was no difference found between the ADSC and ADSC+GCSF groups. In addition, the immunoreactivity of the motor neuron marker, ChAT, in the injured spinal cord was higher in the ADSC+GCSF group than in the other three groups 8 weeks after SCI (Fig. 7).
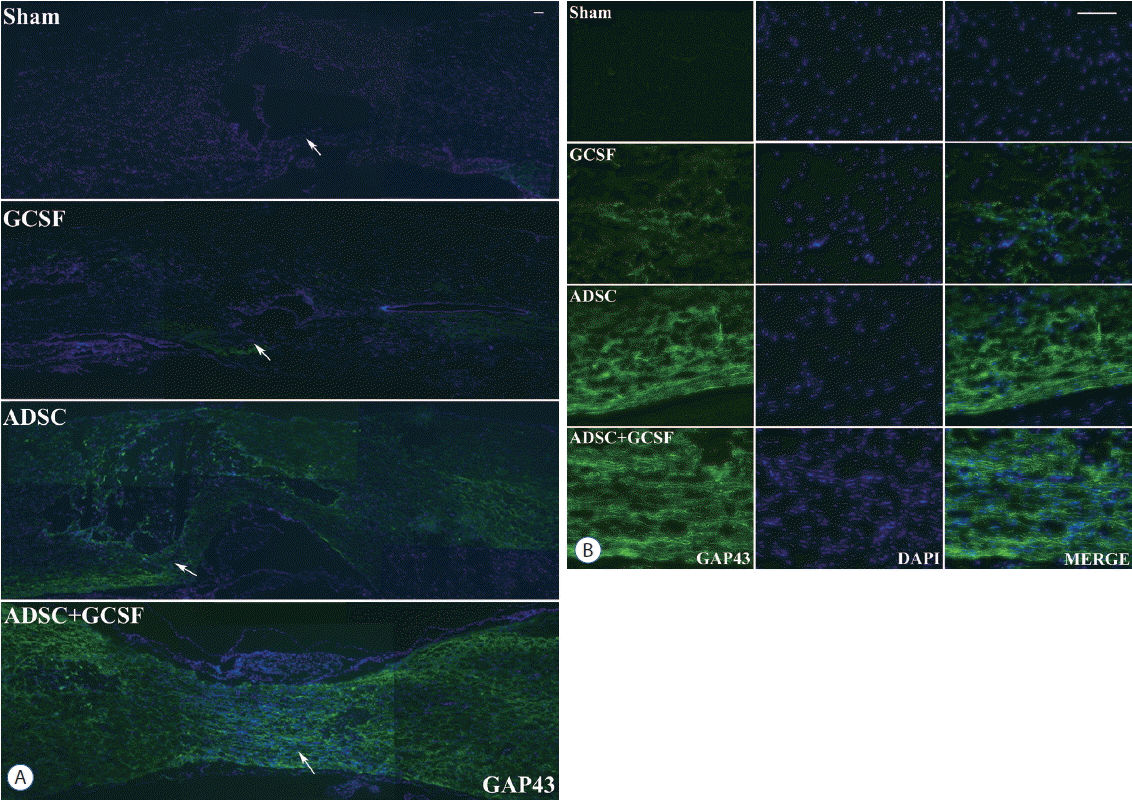
Histological assessment of axonal regeneration using a GAP43. A: Confocal microscopic pictures revealed that anti-GAP43 antibody staining in the spinal cord was greater in the ADSC and ADSC+GCSF groups than in the Sham or GCSF groups 8 weeks after SCI. B: Magnification of white arrow area from (A). Scale bar: 50 μm. ADSC: adipose-derived stem cell, GCSF: granulocyte colony-stimulating factor.
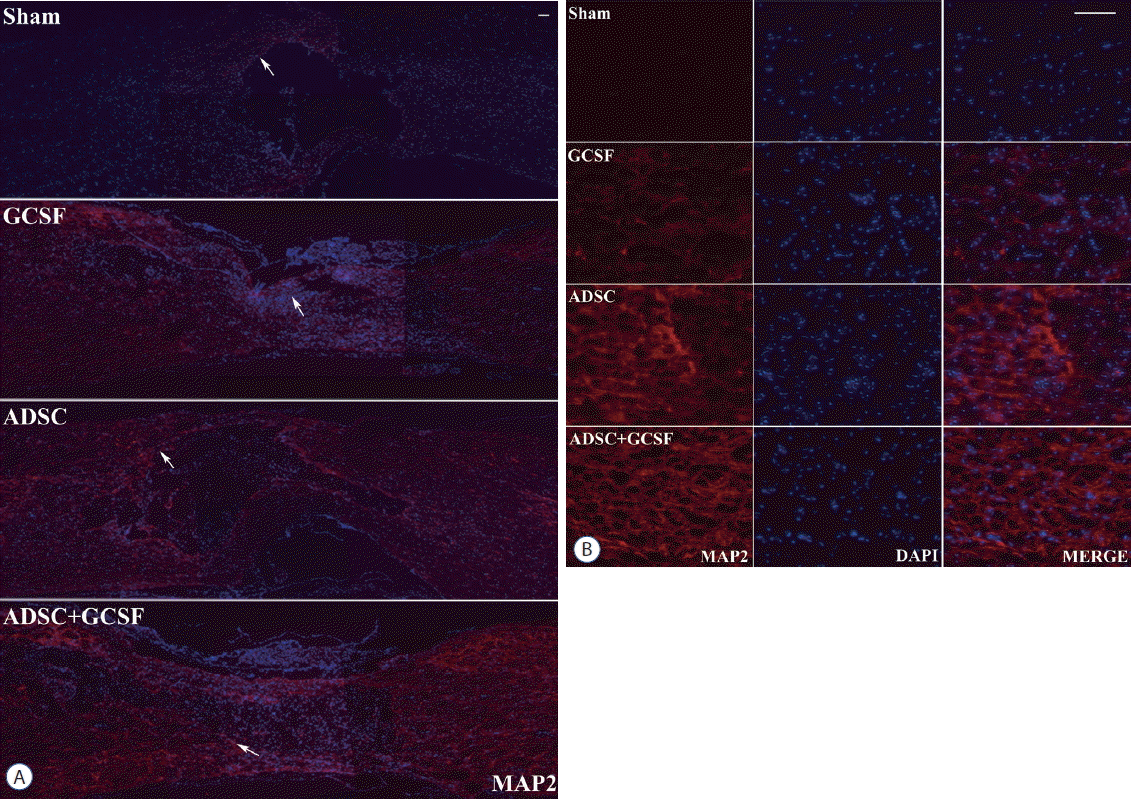
Histological assessment of axonal regeneration using an MAP2. A: Confocal microscopic pictures revealed that anti-MAP2 antibody staining in the spinal cord was greater in the ADSC and ADSC+GCSF groups than in the Sham or GCSF groups 8 weeks after SCI. B: Magnification of white arrow area from (A). Scale bar: 50 μm. ADSC: adipose-derived stem cell, GCSF: granulocyte colony-stimulating factor, SCI: spinal cord injury, DAPI: 4′,6-diamidino-2′-phenylindole.
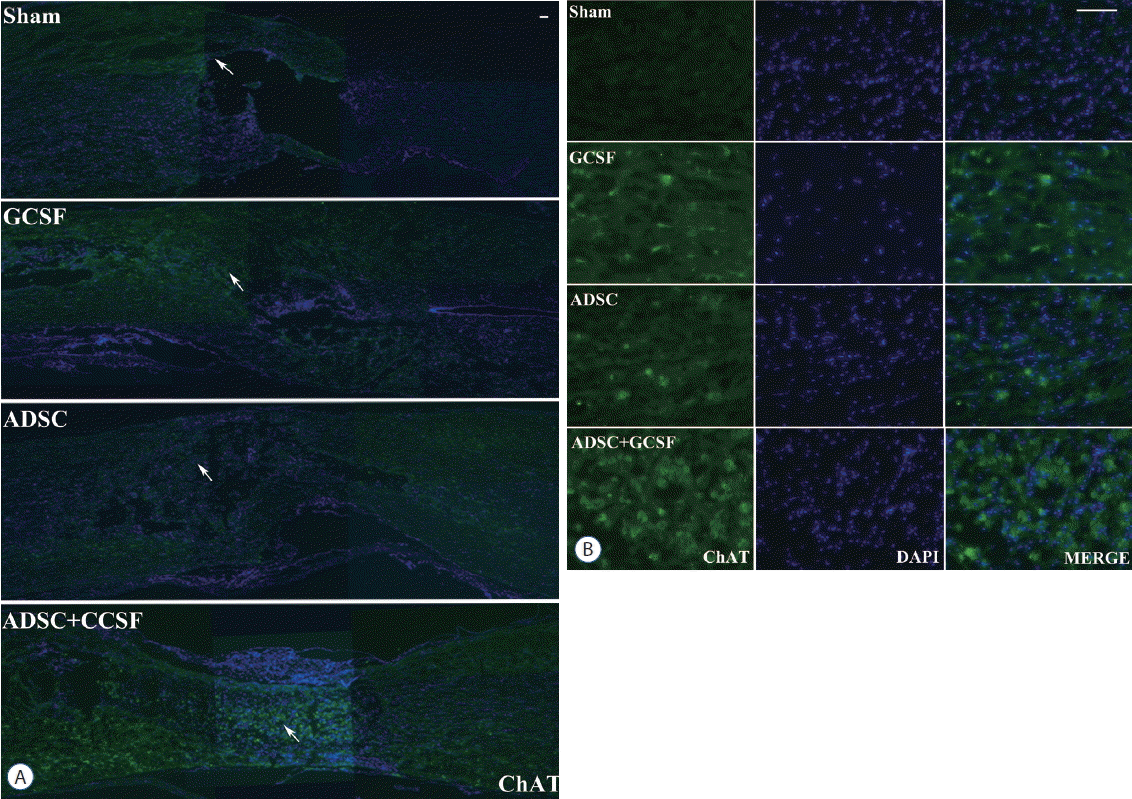
Histological assessment of motor neuron marker using a ChAT. A: Confocal microscopic pictures revealed that anti-ChAT antibody staining in the spinal cord was greater in the ADSC+GCSF group than in other groups 8 weeks after SCI. B: Magnification of white arrow area from (A). Scale bar: 50 μm. GCSF: granulocyte colony-stimulating factor, ADSC: adipose-derived stem cell, SCI: spinal cord injury, DAPI: 4′,6-diamidino-2′-phenylindole.
Western blotting
After western blotting, the relative density levels (RDLs, OD/mm) of the protein bands were measured in all of the SCI groups. For the serotonin transporter, the ADSC group had a significantly higher RDL than the Sham group (2.45±0.07 vs. 2.24±0.08, respectively; p<0.05). The ADSC+GCSF group (2.4±0.03) had a higher RDL than the Sham group, but the difference was not statistically significant (p=0.7). The GCSF group had a lower RDL (2.05±0.03; Fig. 8). For the acetylcholine receptor, the ADSC group had a significantly higher RDL than the Sham group (2.35±0.02 vs. 1.75±0.2; p<0.01), whereas the ADSC+GCSF (2.05±0.03) and GCSF (1.8±0.001) groups had an insignificantly higher RDL than the Sham group (p>0.4; Fig. 9). For GAP43, the three experimental groups particularly the ADSC (0.78±0.001) and ADSC+GCSF (0.76±0.01) groups (p<0.01) had higher RDLs than the Sham group (0.17±0.001; Fig. 10).
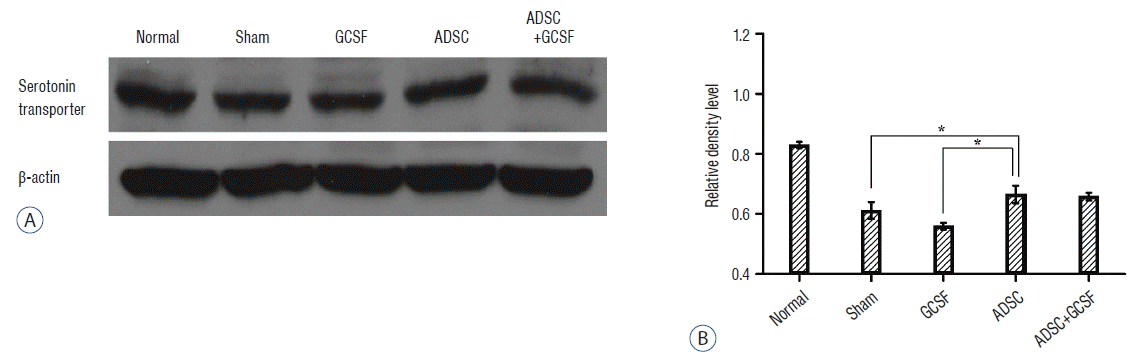
Western blots analysis of serotonin transporter. A: Injured epicenters of 0.5 cm spinal cord were extracted protein and analyzed by Western blots. B: When the band densities were converted into RDL value, the ADSC group showed significantly high density than Sham and GCSF groups. There was no statistical difference of RDL value between the ADSC and ADSC+GCSF groups. *p<0.05. RDL: relative density level, ADSC: adipose-derived stem cell, GCSF: granulocyte colony-stimulating factor.

Western blots analysis of acetylcholine receptor. A: Injured epicenters of 0.5 cm spinal cord were extracted protein and analyzed by Western blots. B: When the band densities were converted into RDL value, the ADSC group showed significantly high density than other groups. *p<0.01. RDL: relative density level, ADSC: adipose-derived stem cell, GCSF: granulocyte colony-stimulating factor.
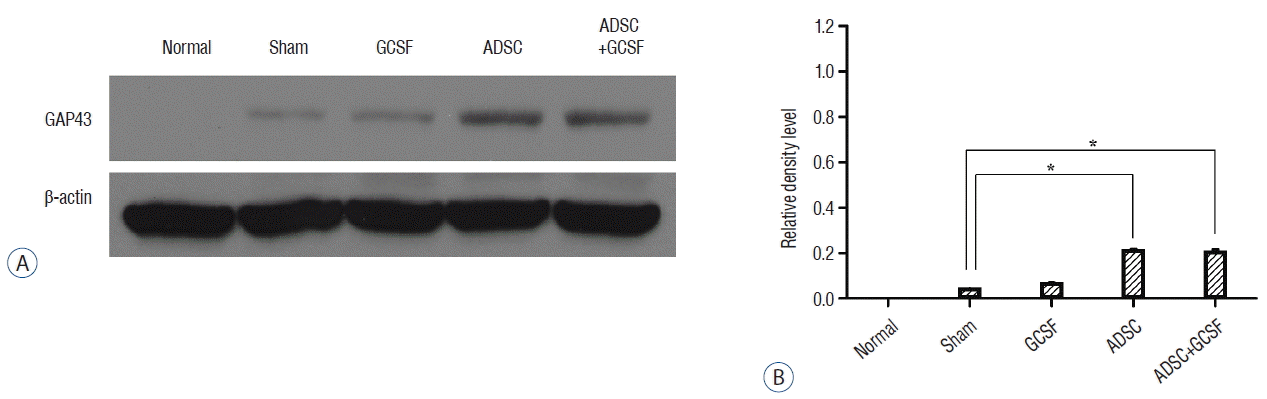
Western blots analysis of GAP43. A: Injured epicenters of 0.5 cm spinal cord were extracted protein and analyzed by Western blots. B: When the band densities were converted into RDL value, the ADSC and ADSC+GCSF groups showed significantly high density than the Sham and GCSF groups. There was no statistical difference of RDL value between the ADSC and ADSC+GCSF groups. *p<0.01. RDL: relative density level, ADSC: adipose-derived stem cell, GCSF: granulocyte colony-stimulating factor.
Reverse transcription polymerase chain reaction (RT-PCR)
RT-PCR results were evaluated in the same way as the western blotting data. For GAP43 gene expression, the ADSC (12.05±0.17) and ADSC+GSCF (16.14±0.06) groups had significantly higher RDLs than the Sham group (7.83±0.11; p<0.01; Fig. 11). However, the GAP43 level of the GCSF group (7.77±0.3) was not significantly different from that of the Sham group. For nerve growth factor (NGF) gene expression, the ADSC (3.38±0.11) and ADSC+GSCF (3.77±0.05) groups had significantly higher RDLs than the Sham group (2.22± 0.08; p<0.01; Fig. 12). However, there was no significant difference between the RDLs of the GCSF (2.19±0.02) and Sham groups.
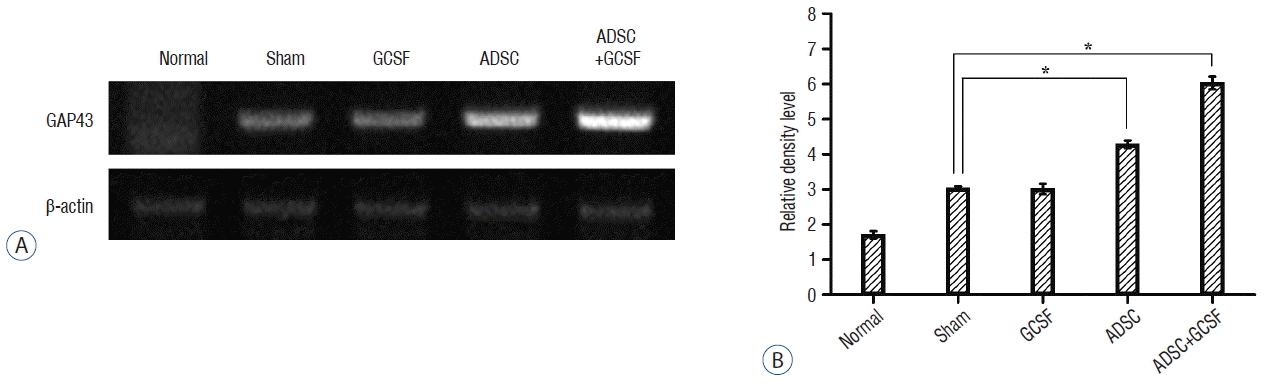
RT-PCR analysis of GAP43. A: Injured epicenters of 0.5 cm spinal cord were extracted mRNA and analyzed by RT-PCR. B: When the band densities were converted to RDL value, the ADSC and ADSC+GCSF groups showed significantly high density than the Sham and GCSF groups. The ADSC+GCSF group showed significantly higher density than ADSC group. *p<0.01. RT-PCR: reverse transcription polymerase chain reaction, RDL: relative density level, ADSC: adipose-derived stem cell, GCSF: granulocyte colony-stimulating factor.
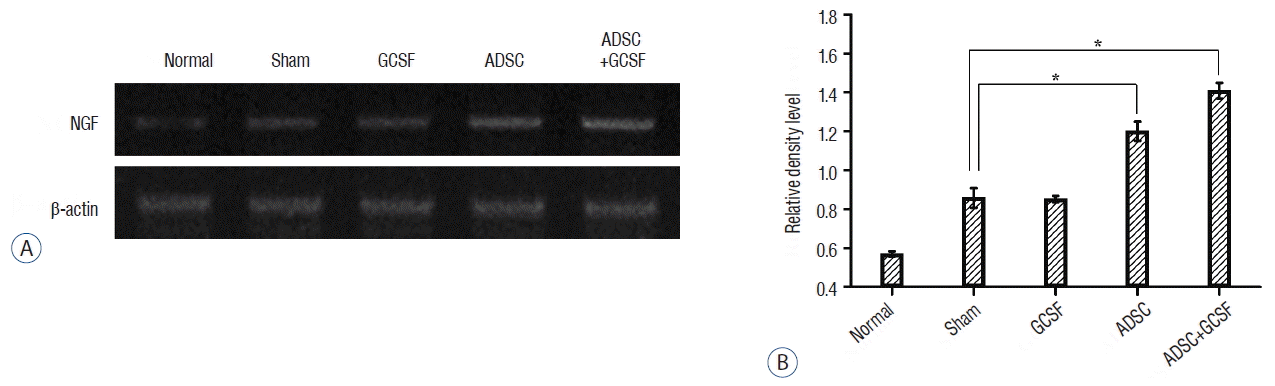
RT-PCR analysis of NGF. A: Injured epicenters of 0.5 cm spinal cord were extracted mRNA and analyzed by RT-PCR. B: When the band densities were converted into RDL value, the ADSC and ADSC+GCSF groups showed significantly high density than the Sham and GCSF groups. The ADSC+GCSF group showed significantly higher density than ADSC group. *p<0.01. RT-PCR: reverse transcription polymerase chain reaction, RDL: relative density level, ADSC: adipose-derived stem cell, GCSF: granulocyte colony-stimulating factor.
DISCUSSION
The rodent injury model is very widely used in the SCI research field42). In our present study, we conducted an animal model of traumatic SCI by using an IH impactor (Precision System and Instrumentation), which can produce more consistent SCI12,39,46) compared to the New York University (NYU) impactor (New York University, New York, NY, USA). The NYU impactor has been a widely used contusion device that generates contusion with weight-drop rod23,28), but it requires an experienced person to generate a successful contusion because there’s a difficulty controlling the impactor. We applied 200 kdyn impacts to create the moderate SCI which is compatible with 10-gram rod drop from 25 mm height by NYU impactor (unpublished data). Moderate SCI is a suitable condition of animal experiment because severe SCI (50 mm height by NYU impactor) might be difficult to manage the animals as high mortality, and mild SCI (12.5 mm height by NYU impactor) is not appropriate to compare the therapeutic effect. Adipose tissue is an abundant tissue in the body and contains a stromal fraction rich in stem cells or progenitor cells. ADSCs can be easily extracted, isolated and capable of undergoing differentiation into osteogenic, chondrogenic, and adipogenic lineages16,42). The ADSCs graft was also known to significantly promote the regeneration of axon compared with bone-marrow derived MSCs as shown in Dr. Zhou’s study with their anterograde axon tracing experiments50).
In recent years, therapeutic effects have been reported in studies using the combined injection method19) suggesting it as the next stage of cell therapy. Through the previous researches, we reviewed a number of therapeutic molecules that had neuroregenerative effects, including GCSF, brain-derived neurotrophic factor, NGF, and vascular endothelial growth factor. Among these molecules, we chose GCSF, which has been shown to induce nerve formation in the central nervous system, increase synaptic plasticity, and inhibit cell death11,18). The administration of GCSF is known to mobilize hematopoietic stem cells from the bone marrow into the peripheral blood9) and it has been used extensively for bone marrow reconstitution and stem cell mobilization25). In addition, the systemic administration of GCSF enhanced the availability of circulating hematopoietic stem cells to the brain, resulting neurogenesis in cerebral ischemia41), and induced neurite outgrowth in hippocampus as well as increased axonal conduction demonstrated by mangan-enhanced magnetic resonance imaging and biotinylated dextran amine tracing32).
GCSF is usually administered systemically (intravenous or subcutaneous) to stimulate bone marrow18,47), and we also injected GCSF via tail vein to achieve the same mechanism. On the contrary, stem cells are administered through a local application such as intramedullary or intrathecal injections because stem cells function locally including filling the contused cavity mechanically or forming a matrix to bridge for axon regeneration through the cavity as we reported previously30,43). Also, intrathecal injection of stem cells is a clinically well-established method20,29,30). Therefore, we think intravenous GCSF injection with intrathecal stem cell administration is most appropriate to verify the effectiveness of combined application and designed the study using this method.
In previous studies, GCSF is used in a dose range from 0.5–5 μg/100 g systemically in rats for the treatment of contusive SCI18,47). In this study, we performed injection of GCSF (5 μg/100 g) via tail vein which is the most common and maximal dose reported for SCI study. GCSF dose in this range was confirmed to show higher expression of angiogenic cytokines, sparing of white matter and significantly greater recovery of hindlimb function after SCI18,47).
In the studies of rodent SCI, three to seven days after SCI seem to be the optimal time window to apply cell therapy14,27,33,34). In addition, the effect of stem cell was demonstrated even in the chronic status of SCI in human patients15,30). The chronic phase of SCI in rat is defined as the period since seven days after SCI22,31,44). In this study, we intended to apply therapeutic materials in the period covering all effective time windows. Therefore, we injected GCSF and ADSCs 3, 7, and 14 days after SCI.
For immunofluorescent evaluation, we used several markers including GAP43, MAP2, and ChAT to evaluate axon regeneration and neuronal preservation. GAP43, which is expressed during the re-growth of axon growth cone membrane50), can be utilized as an axon regeneration marker. The MAP2 which is involved in microtubule assembly, an essential step in neurogenesis, could also be utilized19). ChAT was used as an immune staining marker for motor neurons, which is synthesized within the body of a neuron and joins Acetyl-CoA to choline48). Also, we were trying to measure the quantity of neurotransmitters by the western blot method of acetylcholine receptor and serotonin transporter. Acetylcholine receptor is transmembrane receptor that responds to binding of acetylcholine which is one of the many neurotransmitters in the autonomic nervous system, but the only neurotransmitter used in the motor division of the somatic nervous system41). In addition, serotonin transporter is an integral membrane protein that transports the neurotransmitter serotonin from synaptic spaces into presynaptic neurons32). The levels of GAP43 were found to be significantly increased in the cell injection groups (ADSC and ADSC+GCSF groups), as seen from our current immunofluorescent study, western blotting, and RT-PCR results. The presence of this protein could indicate the new growth of axons, which may have enabled the functional recovery witnessed in the behavioral tests, in response to the repeated injection of ADSCs. However, the additive effect of the GCSF on GAP43 levels was not observed to be significant because it increased both in ADSC and ADSC+GCSF groups and there was no significant difference between them like the result of BBB locomotor rating scale. The results of serotonin transporter level in western blot and the level of NGF in RT-PCR were found to be increased in ADSC and ADSC+GCSF groups higher than those of Sham and GCSF groups. However, there was also no significant difference between ADSC and ADSC+GCSF group, which also implies no additive effect of combination therapy.
CONCLUSION
Therefore, in our present study, we could not find additive effects of GCSF in combined ADSCs treatment for acute SCI although GCSF with its reported functions, as we described previously, is expected to exert additive effect when working with stem cells. One previous study which evaluated the combination effect of umbilical cord blood-derived MSCs and GCSF on a canine SCI model also reported no beneficial effect of GCSF when treated with UBC-derived MSCs. They suspected the possibility of a down-regulation of MSCs proliferation by GCSF5) since GCSF was known to enhance stem cell damage when it combined with certain cytotoxic agents29). However, there was no cytotoxic agent in this study and harmful effect of GCSF was also not observed. We think the results would be similar even if the GCSF had been applied in the same way as the cells because GCSF effects start from bone marrow stimulation as we mentioned above. GCSF intrathecally injected would be absorbed systemically and stimulate bone marrow. The reasons why additive therapeutic effects of these two molecules were not exerted should be investigated, and molecules other than GCSF or a modification of the methodology should be considered in further study for combination therapy with stem cells.
Acknowledgements
This study was supported by a grant (12-176) from the Asan Institute for Life Sciences, Seoul, Korea.
This study was reviewed and approved by the IACUC Asan Institute for Life Sciences, Asan Medical Center. The committee abides by the ILAR guide (2012-02-029).