Protected versus Unprotected Carotid Artery Stenting : Meta-Analysis of the Current Literature
Article information
Abstract
Objective
To compare peri-operative any symptomatic stroke after carotid angioplasty and stenting (CAS), based on the application or absence of a cerebral protection device.
Methods
A systematic literature review using PubMed, Embase, and the Cochrane Central was done across an online data base from January 1995 to October 2016. Procedures which were performed due to carotid dissection or aneurysm, procedures using covered stents or conducted in an emergency, were excluded. The primary endpoint was perioperative any symptomatic stroke within 30 days after the procedure. A fixed effect model was used in cases of heterogeneity less than 50%.
Results
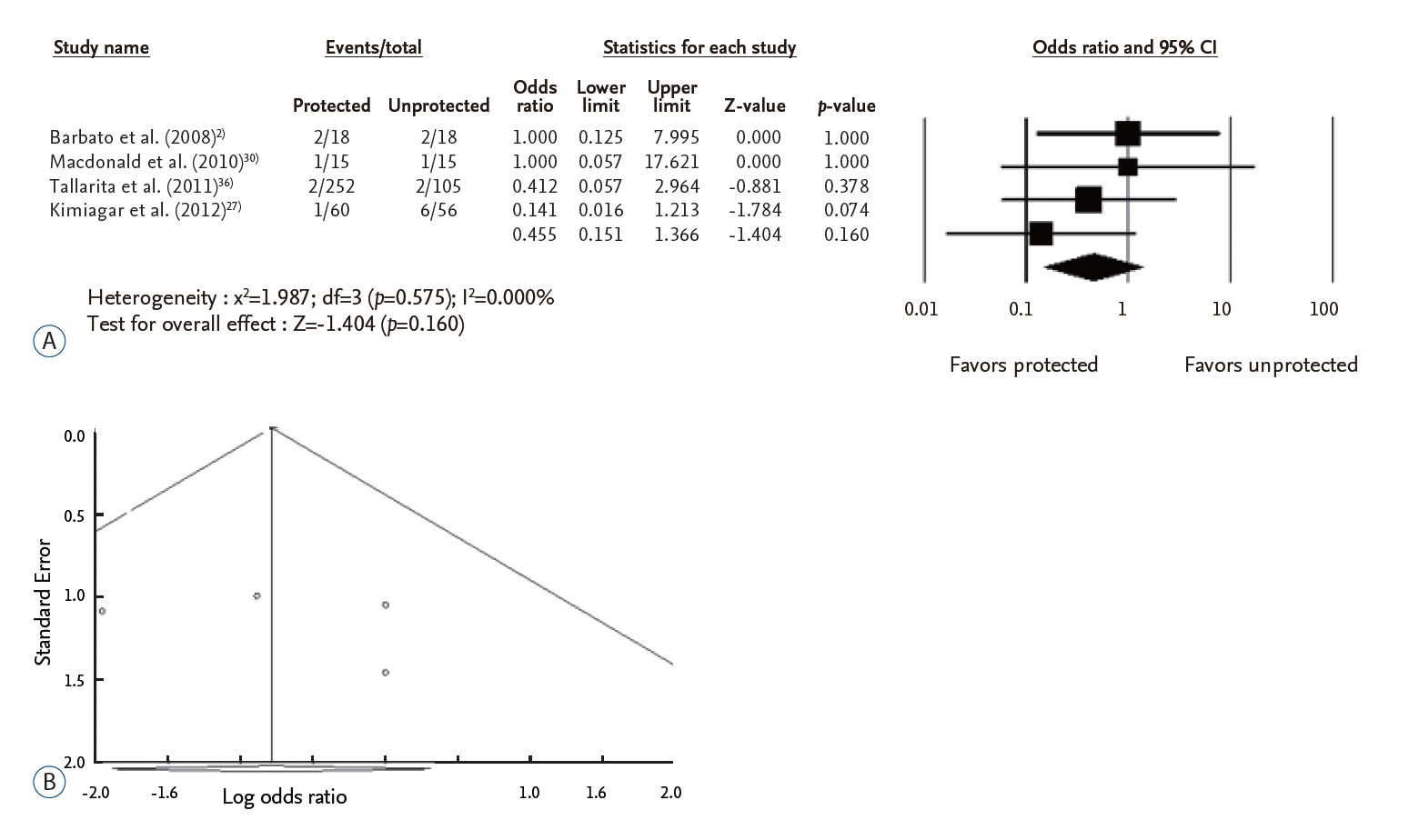
In the 25 articles included in this study, the number of stroke events was 326 (2.0%) in protected CAS and 142 (3.4%) in unprotected CAS. The use of cerebral protection device significantly decreased stroke after CAS (odds ratio [OR] 0.633, 95% confidence interval [CI] 0.479–0.837, p=0.001). In the publication bias analysis, Egger’s regression test disclosed that the intercept was -0.317 (95% CI -1.015–0.382, p=0.358). Regarding symptomatic patients (four studies, 539 CAS procedures), the number of stroke was six (1.7%) in protected CAS and 11 (5.7%) in unprotected CAS. The protective effect against stroke events by cerebral protection device did not have a statistical significance (OR 0.455, 95% CI 0.151–1.366, p=0.160).
Conclusion
The use of protection device significantly decreased stroke after CAS. However, its efficacy was not demonstrated in symptomatic patients. Routine use of protection device during CAS should be critically assessed before mandatory use.
INTRODUCTION
Distal cerebral protection devices have been widely used during carotid angioplasty and stenting (CAS), to reduce thromboembolic complications. However, there are concerns of possible thromboembolic events during the placement of protection device in patients with tortuous carotid artery, near-occlusion of carotid artery, or thrombus in stenotic area [36]. In some patients, stroke after CAS occurs despite the use of protection device. Although previous studies, including systematic reviews [12,40], showed the efficacy of protected device in reducing perioperative complications after CAS, some studies have doubted the real effectiveness of protection device [2,30,36]. Two studies recently reported and exhibited disagreement regarding the efficacy of protection device [15,19].
Meta-analysis of treatment outcome between CAS and carotid endarterectomy (CEA) has been updated to assess the treatment efficacies [45]. However, a systematic review and meta-analysis of treatment outcome between protected and unprotected CAS has not been reported since 2009, although a number of studies have been continuously published. Here, we conducted a meta-analysis to evaluate perioperative stroke after CAS, based on whether or not a cerebral protection device was used.
MATERIALS AND METHODS
Search strategy
Core search between January 1990 and October 2016 through PubMed, Embase, and the Cochrane Central was done, using the key words “carotid stenosis”, “stents”, “balloon”, “carotid angioplasty”, “percutaneous transluminal angioplasty”, “stroke”, “myocardial infarction”, “death”, “perioperative complications”, and “mortality” [12,40].
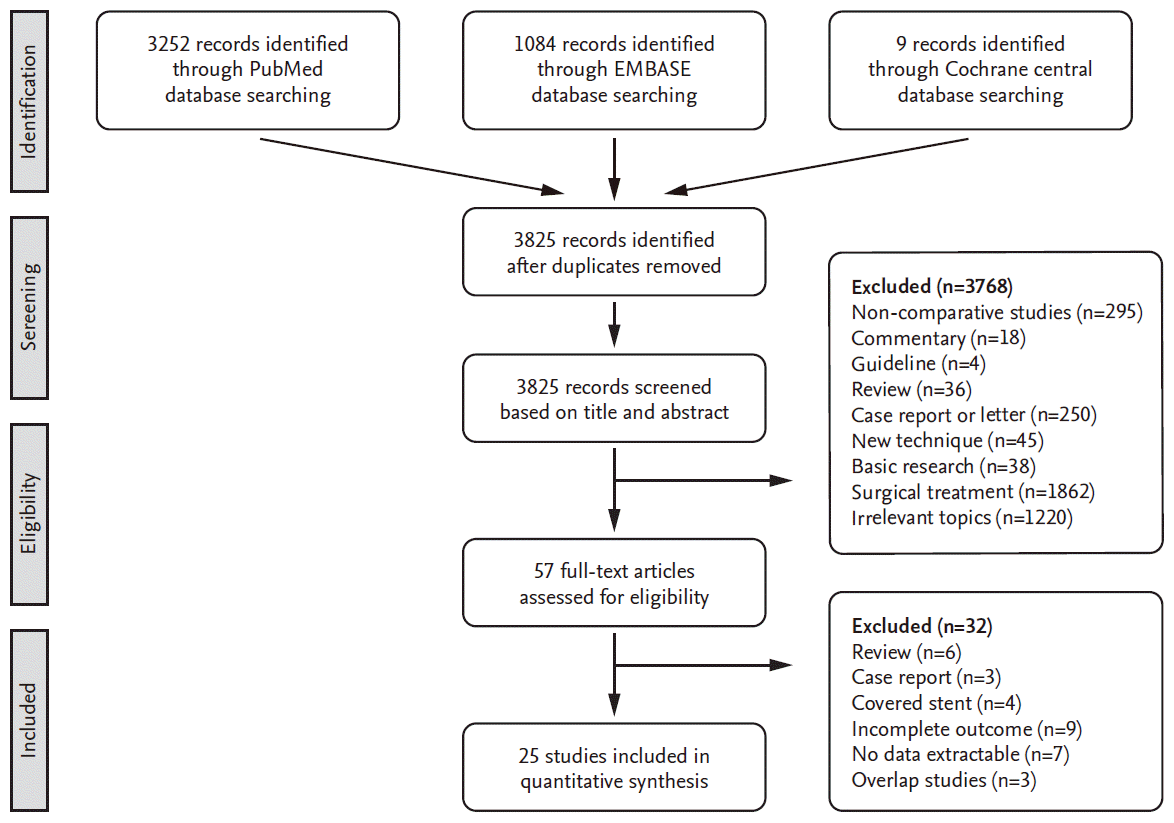
Our criteria for inclusion in this study were : 1) symptomatic and/ or asymptomatic stenosis in the internal carotid artery or carotid bifurcation, 2) CAS procedures with or without cerebral protection through common femoral artery over 20 [12], and 3) the number of peri-procedural complications such as stroke, death or myocardial infarction within 30 days was reported separately through a comparative study between protected and unprotected CAS [40]. Stroke was defined as any sudden neurologic deficits due to cerebral infarction [39] including bilateral involvement. Asymptomatic signal change on brain MRI was not included for this meta-analysis. In case of overlap, the most recently published article was selected for analysis. Assessing risk of bias in included studies was performed using Cochrane risk of bias for randomized controlled studies (Supplementary Fig. 1) and Newcastle-Ottawa scale for nonrandomized studies. The exclusion criteria included : 1) incomplete data or unclear distinction between protected and unprotected CAS, 2) review articles or case reports, 3) procedures due to dissection, carotid aneurysm, urgently conducted procedure or the use of covered stents, and 4) other procedural approach, other than common femoral artery [12,23,34].
Data extraction
An extensive electronic search was performed by an experienced researcher. Then articles were reviewed and selected on specific criteria by two investigator (J.P.J. and Y.S.K.) followed by discussion. Disagreements between the two authors were resolved by discussion and consultation with a third author. The primary endpoint was perioperative any symptomatic stroke within 30 days after the procedure [40]. Subgroup analyses were performed only for symptomatic carotid stenosis. This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Statistical analysis
Dichotomous variables are presented as odds ratio (OR) with a 95% confidence interval (CI). Heterogeneity was evaluated by using the I2 test. If I2 was less than 50%, a fixed effect model was used [21]. Publication bias was determined using Begg’s funnel plot and Egger’s test of the intercept [3,11,22]. Comprehensive meta-analysis (CMA) software (CMA v2.2.064, Biostat, Englewood, NJ, USA) was used for all the above, with statistical significance indicated at p<0.05.
RESULTS
Identification of relevant studies
Fig. 1 displays a flow diagram of the detailed search process. After screening the records and deciding eligibility, 25 articles were included (Table 1). At the subgroup analysis, four studies included the data of symptomatic carotid stenosis.
Comparison of the perioperative stroke between protected and unprotected CAS
A total of 20670 CAS procedures from 25 studies were included in this analysis (Fig. 2A). Among them, 16440 procedures were done with cerebral protection device and 4230 were done without protection device. The number of stroke was 326 (2.0%) in protected CAS and 142 (3.4%) in unprotected CAS. The use of cerebral protection device significantly decreased stroke after CAS (OR 0.633, 95% CI 0.479– 0.837, p=0.001). In the publication bias analysis for comparison between protected and unprotected CAS, Egger’s regression test disclosed that the intercept was -0.317 (95% CI -1.015 –0.382, p=0.358). Accordingly, there was no evidence of publication bias in this comparison (Fig. 2B).
Comparison of the perioperative stroke in symptomatic carotid stenosis
A total of 539 CAS procedures from four studies were included in this analysis (Fig. 3A). Of theses, 345 procedures were done with cerebral protection device and 194 were done without protection device. The number of stroke was six (1.7%) in protected CAS and 11 (5.7%) in unprotected CAS. The use of cerebral protection device did not decrease the events of stroke after CAS (OR 0.455, 95% CI 0.151–1.366, p=0.160). In the publication bias analysis for comparison between protected and unprotected CAS, Egger’s regression test disclosed that the intercept was 1.6592 (95% CI -13.600–16.918, p=0.686). Accordingly, there was no evidence of publication bias in this comparison (Fig. 3B).
DISCUSSION
Although many reports are available, the efficacy of protection device in preventing thromboembolic complications during CAS remains inconclusive. Our study showed that using cerebral protection device significantly lowered the stroke. However, its efficacy was not demonstrated in symptomatic lesions.
During the delivery of protection device, thromboembolic complications can occur while passing over the severe stenotic lesions or vulnerable plaque. In addition, protection device sometimes cannot be deployed at the destination site due to the stiffness in the tortuous or kinked carotid artery [36]. Subsequently, the efficacy of protection device should be assessed by an updated knowledge, although protection devices are widely accepted for the procedure.
Garg et al. [12] compared total stroke events within 30 days after the procedures between protected and unprotected CAS. They concluded that protected CAS showed a relative risk reduction of 0.59 (95% CI 0.47– 0.73) than unprotected CAS, in 24 studies. Through a systemic review, Touzé et al. [40] reported a 4.7% (95% CI 4.1– 5.2) reduction within the 30-day risk of stroke or death rate after CAS. In their study [40], the protection device lowered the periprocedural complications with risk reduction of 0.57 (95% CI 0.43–0.76). Our study also showed that cerebral protection device significantly decreased the events of stroke. However, substantial heterogeneity across the studies can be a concern to interpret the results of previous meta-analysis [40]. In addition, only two randomized controlled trial (RCT) studies [31,35] were enrolled in their investigations. Analyzing three RCT studies, the Cochrane review [5] reported that the number of either stroke or death within 30 days after CAS did not differ significantly, based on the use of protection device (OR 0.95, 95% CI 0.38–2.41) [20,31,35]. In this meta-analysis, only two studies [2,30] provided clear information on stroke and death, respectively, not sum of stroke and death. That was because most previous RCTs have compared treatment outcomes between CAS and CEA, not focusing on the use of protection device. Accordingly, further analysis of individual patient data are necessary.
Symptomatic stenosis affects the periprocedural risk after the procedure. A systemic review [40] showed that symptomatic lesion increased the 30-day risk of stroke or death, more than asymptomatic lesions (7.6%, 95% CI 6.3–9.1 vs. 3.3%, 95% CI 2.6–4.1). Garg et al. [12] also reported that symptomatic patients had a higher stroke rate than asymptomatic patients, comparing patients who underwent protected (3.8% vs. 1.7%) and unprotected CAS (5.6% and 2.8%). For symptomatic patients, the protection device exhibited relative stroke risk reduction of 0.67 (95% CI 0.5–2 0.86). Kosowski et al. [28] compared the long-term adverse events between symptomatic and asymptomatic patients who underwent CAS. The risk of stroke or death did not differ significantly between symptomatic (8.3%) and asymptomatic patients (8.6%). In this study, we did not find a significant difference in the number of stroke between protected (n=6, 1.7%) and unprotected CAS (n=11, 5.7%) in symptomatic patients (OR 0.455, p=0.160). We think that difference in the primary endpoint (stroke vs. stroke and death) resulted to the disagreement. Accordingly, further large scale RCT studies are required to investigate the periprocedural risk according to the use of protection device, including symptomatic stenosis.
Technical differences in stent type and protection device are related to the periprocedural complications after CAS. The procedures are performed using various stents with different cell designs. Bosiers et al. [6] reported that the postprocedural event rate was more pronounced in open cells (3.4%) than closed cells (1.3%), in particular in symptomatic patients. However, a recent meta-analysis by Kouvelos et al. [29] did not show the risk reduction of death (OR 0.69, p=0.21) and stroke (OR 1.17, p=0.37) according to cell design, within 30 days after the procedure. Cerebral protection can be conducted by balloon occlusion of the internal carotid artery above the stenotic lesion, filter instrument and flow-reversal system [18]. Embolic events are more found in filters than proximal occlusion or flow reversal system while crossing the lesion [18]. Thus theoretically, proximal embolic protection device can be advantageous in preventing stroke during CAS [14]. Giri et al. [14] compared the clinical outcome between distal and proximal protection devices during CAS. In their study, the 30-day adverse events did not reach significance according to the device types (p=0.07). Zhan et al. [43] also reported that in-hospital stroke or death did not differ significantly between filter (10 out of 551, 1.8%) and distal occlusive (4 out of 176, 2.3%) embolic protection device (OR 1.04, 95% CI 0.24– 4.44, p=0.958). Nevertheless, future prospective trials comparing stent design and protection device properties are needed.
There are some limitations in this study. First, most studies of this investigation did not analyze the efficacy of the protection device according to the symptomaticity. Second, two out of the 25 studies (8%) are RCTs, although a number of studies have drawn their conclusion from a prospective registry. Third, heterogeneity in terms of primary endpoints (stroke [12] vs. stroke or death vs. stroke and death) can be a limitation to reach the conclusion in the previous studies. In addition, some studies did not provide clear information on stroke, death, and their summation, respectively. Accordingly, total events can be overestimated because major stroke can be fatal, although total events were estimated as the sum of any stroke or death in previous study [25]. Accordingly, randomized controlled studies including more detailed data on perioperative complications according to the symptomaticity and risk stratification, and adverse events in long-term observation are required.
CONCLUSION
Our meta-analysis showed the use of cerebral protection device significantly decreased any symptomatic stroke after the CAS. However, its efficacy was not demonstrated in symptomatic patients. Therefore, routine use of protection device during CAS should be critically assessed before mandatory use.
Notes
No potential conflict of interest relevant to this article was reported.
INFORMED CONSENT
This type of study does not require informed consent.
Acknowledgements
This research was supported by a grant (PJ01121401) from BioGreen 21 of the Rural Development Administration, and partly by National Research Foundation of Korea grant funded by the Ministry of Science, Information and Communication Technologies and Future Planning of the Korea Government (No. 2017M3A9E8 033223).
Supplementary Materials
The online-only data supplement is available with this article at https://doi.org/10.3340/jkns.2017.0202.001.