Multilevel Percutaneous Vertebroplasty (More than Three Levels) in the Management of Osteoporotic Fractures
Article information
Abstract
Objective
Percutaneous vertebroplasty (PV) is a minimally invasive procedure designed to treat various spinal pathologies. The maximum number of levels to be injected at one setting is still debatable. This study was done to evaluate the usefulness and safety of multilevel PV (more than three vertebrae) in management of osteoporotic fractures.
Methods
This prospective study was carried out on consecutive 40 patients with osteoporotic fractures who had been operated for multilevel PV (more than three levels). There were 28 females and 12 males and their ages ranged from 60 to 85 years with mean age of 72.5 years. We had injected 194 vertebrae in those 40 patients (four levels in 16 patients, five levels in 14 patients, and six levels in 10 patients). Visual analogue scale (VAS) was used for pain intensity measurement and plain X-ray films and computed tomography scan were used for radiological assessment. The mean follow-up period was 21.7 months (range, 12–40).
Results
Asymptomatic bone cement leakage has occurred in 12 patients (30%) in the present study. Symptomatic pulmonary embolism was observed in one patient. Significant improvement of pain was recorded immediate postoperative in 36 patients (90%).
Conclusion
Multilevel PV for the treatment of osteoporotic fractures is a safe and successful procedure that can significantly reduce pain and improve patient’s condition without a significant morbidity. It is considered a cost effective procedure allowing a rapid restoration of patient mobility.
INTRODUCTION
Percutaneous vertebroplasty (PV) is a minimally invasive procedure in which bone cement is injected into the vertebral body. This technique was first performed by Deramond and Galibert in 1987 for the treatment of vertebral hemangiomas [20]. Since that time, PV gains a worldwide popularity and was used in the management of various spinal pathologies as metastasis and osteoporosis. With the increase of the aging population all over the world, osteoporotic fractures become very common in the daily practice [16].
PV is very efficient technique in reducing pain associated with osteoporotic fractures resulting from the local chemical, vascular and thermal analgesic effect of polymethylmethacrylate (PMMA) on nerve endings of surrounding tissues. In addition, it allows immediate stabilization of the fractured bone by the bone cement [9,13,34]. Patients with osteoporotic fractures can face a single or more commonly a multilevel affection. Still no agreement about the maximum levels to be injected at one session [16].
MATERIALS AND METHODS
Between June 2010 and May 2015, all patients who underwent multilevel PV (more than three levels) for osteoporotic compression fractures were collected. There were 28 females and 12 males and their ages ranged from 60 to 85 years with mean age of 72.5 years. All patients were subjected to detailed history taking and neurologic examination. As regards the clinical presentation, all patients had presented with back pain before surgery. All patients had preoperative plain X-ray, computed tomography (CT) and magnetic resonance imaging (MRI) examination. Vertebral collapse and MRI signal changes in the short tau inversion recovery (STIR) sequence were used to determine the number of vertebrae requiring vertebroplasty in our patients. Bone marrow oedema detected by STIR sequence of MRI was used to differentiate between healed and non-healed fractures. Patients with osteoporotic fractures were followed side by side by internal medicine doctors for treatment of osteoporosis. Calcitonin-salmon (Miacalcin) nasal spray, calcium and vitamin D supplements, bisphosphonates as well as wellbalanced diet, regular exercise program, smoking cessation were provided for all osteoporotic patients. Physical therapy, back braces, muscle relaxants and analgesics were used to control pain.
Inclusion criteria
Patients with painful multilevel (more than three levels) osteoporotic vertebral compression fractures without radiological evidence of neural compression and who showed no response after medical treatment or progression of vertebral fracture. Patients with less than three osteoporotic vertebral compression fractures but where prophylactic vertebroplasty was performed for borderline adjacent vertebrae which showed bone marrow oedema in the STIR sequence of MRI. Patients with recent and old symptomatic osteoporotic fractures were included in the study.
Exclusion criteria
Patients with osteoporotic fractures requiring injection in three levels or less and fractures due to neoplastic pathology. Patients with coagulation disorders or any local or systemic infection. Destruction of the posterior vertebral cortex as well as decreased vertebral height were not considered exclusion criteria.
Surgical technique
PV using the PMMA was performed under local anesthesia with light sedation in all our patients. Patients were placed in prone position with abdominal and thoracic support to allow adequate ventilation. A completely sterile technique was used. The transpedicular approach was performed for all our thoracic and lumbar vertebral fractures. The transpedicular needle was introduced in the anterior third of the vertebral body. The cement mixture was then injected gradually through the needle with careful fluoroscopic control in both anteroposterior and lateral views. Adequate consistency of bone cement was required before injection to decrease the incidence of the leakage. Cement leakage was detected during injection with immediate cessation when it happened and then restarted again after one minute. A unilateral transpedicular injection was used in most of our patients. In the cases for whom unilateral injection was not satisfactory or for whom there was a need for both pedicles to be stabilized, a bipedicular approach was used.
We had injected 194 vertebrae in those 40 patients (four levels in 16 patients, five levels in 14 patients, and six levels in 10 patients) (Figs. 1 and 2). Vertebroplasty included 140 lumbar vertebrae and 54 dorsal vertebrae. Vertebroplasty was performed for 124 (64%) fractured vertebrae while prophylactic injection was done in 70 borderline vertebrae (36%). Unilateral transpedicular injection was done in 124 vertebrae.
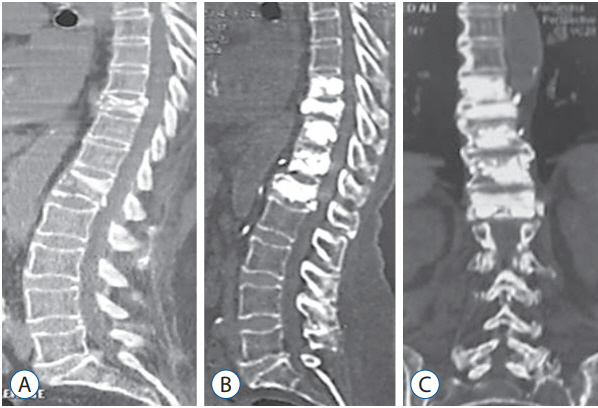
CT scan (sagittal view) showing a D10 and L1 osteoporotic fractures in a 73 years old female patient (A). Postoperative CT scan follow-up after one year (sagittal and coronal cuts) showing multilevel percutaneous vertebroplasty from D9 to L1 with good restoration of the vertebral heights after vertebroplasty (B and C). CT : computed tomography.
Postoperative care and follow up
Postoperatively, all patients were neurologically examined with assessment of motor power. Visual analogue scale (VAS) was used for pain intensity measurement and plain X-ray films (AP and lateral views) and CT scan were used for radiological assessment in all our patients. Patients were evaluated regularly immediate post vertebroplasty and then every 6 months both clinically and radiologically. Spinal MRI was performed if any neurological deficit appears or if the back pain increases in severity during the follow up. The mean follow-up period was 21.7 months (range, 12–40) including both clinical and radiological examinations.
Statistical analysis of the data
Data were analyzed using IBM SPSS software package version 20.0 (IBM SPSS Inc., Chicago, IL, USA). Qualitative data were described using number and percent. Quantitative data were described using range, mean, standard deviation and median. For ordinal data to compare between the different periods, Wilcoxon signed ranks test was applied. Significance of the obtained results was judged at the 5% level.
RESULTS
PV was performed for osteoporotic vertebral compression fractures in 54 dorsal and 140 lumbar vertebrae in the present study. The most common injected levels were D12 and L1 vertebrae injected in 34 patients each followed by L2 level injected in 32 patients (Table 1). The least levels injected were D7, D8, and D9 found in two patients each. The average amount of bone cement injected was 5.6 mL in the lumbar spine and 4.3 mL for the dorsal spine. MRI was performed in one patient in whom back pain had increased in intensity after vertebroplasty without recent CT explanation. His MRI was insignificant and good response to analgesic was observed later on.
Bone cement leakage
Asymptomatic bone cement leakage has occurred in 12 patients (30%); leakage in the disc space in six patients and paravertebral leakage in the other six patients (Fig. 3). All those patients were doing fine and no further management was required. Symptomatic pulmonary embolism was observed in one patient without an evident cement leakage. This patient has presented with dyspnea postoperatively and required intensive care unit (ICU) admission and received anticoagulant with satisfactory recovery. None of the patients developed discitis or epidural leakage.
Back pain
The VAS was used to assess the degree of back pain improvement after PV. The VAS was assessed immediate postoperative and then every six months. The preoperative low back pain VAS mean value was 8.25. Immediate postoperatively the VAS dropped to 3.45 and after 6 month it was 2.33 and after 1 year it was 2.2 (Table 2, Fig. 4). Significant improvement of pain (defined as decrease of VAS scores of more than 50%) was recorded immediate postoperative in eighteen patients (90%) (p<0.001). Better improvement than the postoperative baseline in later follow up was also observed.
Refractures
No further vertebral collapse was found in the injected vertebrae. Four out of 40 patients (10%) developed a new fracture and required a second vertebroplasty. Two of them had fracture adjacent to vertebroplasty levels while in the other two, the fracture was distant.
DISCUSSION
PV was first performed in France and then introduced into the United States and the whole world. It was used in the management of metastatic deposits and hemangiomas. Recently, osteoporotic compression fractures are efficiently treated with PV [5,8,13]. PV in osteoporotic fractures allows an effective pain reduction, early ambulation with lower complications as compared with open vertebroplasty. Also PV guards against further spinal deformity by solidifying fracture lines and restoring the bony space lost by the fracture, thus providing immediate structural support. This point can’t be obtained with medical treatment [30].
In the present study, we included the cases that required more than three levels injection. The minimum levels injected were four and the maximum were six. Till now, the maximum number of vertebrae that can be injected at one session is still debatable. Barr et al. [3] have stated that single level injection is associated with better outcomes than multiple levels. Other studies suggest doing no more than three levels injection at one session to reduce the complications associated with PV as well as patient discomfort [16]. Zoarski et al. [35] mentioned that up to five levels injection is acceptable and treatment of eight levels or more simultaneously is not accepted medical practice. Mailli et al. [20] found no statistical difference when comparing PV performed up to three vertebrae with more than three levels per session. This study included patients with osteoporosis, metastasis and hemangioma. They measured the degree of pain reduction and improved mobility in the follow up. They concluded that the results of PV are not dependent upon the number of vertebrae injected per session and that PV is an efficient and safe technique with multilevel injection. This was also concomitant with what was reported by Singh et al. [25] and Anselmetti et al. [2] who reached the same conclusion of performing vertebroplasty in multiple levels with safe and effective results.
Prophylactic cement injection into the vertebral body adjacent to the fractured vertebra was performed in 70 vertebrae (36%). This technique was highly recommended by some authors to prevent new fractures after PV [15]. However others [4,12] concluded that this procedure did not lower the risk of recurrence and that prediction of vertebrae at risk is difficult. The STIR sequence of MRI is very sensitive in detecting vertebral edema due to fresh fractures or micro-fractures in osteoporotic patients which is of great value to select the targeted vertebrae in multilevel PV. This will justify the risk and cost of prophylactic injection of adjacent or distant vertebrae to prevent fractures and refractures with minimal complications [16,28].
The present study showed a significant pain improvement (defined as decrease of VAS scores of more than 50%) in 90 % of the patients. Similarly, Mailli et al. [20] who studied the outcome and safety of multilevel PV found a significant pain reduction in 96.9% of their patients. The pain relief was obtained within 24 hours after the procedure and remained unchanged throughout the 2-year follow-up period. Also, Grados et al. [10] had reported a decrease of VAS from 8.0 to 3.7 after 6 months in their patients with osteoporotic fractures treated by vertebroplasty. McGraw et al. [21] reported that 97% of their patients who had undergone a PV showed a significant pain improvement after 24 hours. Similarly, other studies of PV for osteoporotic patients that used the VAS as an evaluation method showed similar results in pain improvement [25,33,35].
In the present study the overall incidence of cement_leakage was 30% however all were asymptomatic. Mailli et al. [20] showed a similar incidence (33.8%) of cement leakage in their osteoporotic patients treated with PV. Layton et al. [17] who published a series of 1000 cases of PV in 552 patients reported a 1.8% clinical significant complications and 25% clinical insignificant leakages.
Leakage of bone cement into the disc space is not uncommon during vertebroplasty and usually with no clinical significance [19]. Fifteen percent of our cases showed asymptomatic cement leakage into the disc space. Increased fracture risk of adjacent vertebral bodies had been mentioned by some authors following cement leakage into the disc space however not fully documented [19]. Proper needle position and cement viscosity as well as immediate cessation of injection after any leakage reduce this event.
The incidence of paravertebral cement leakage varies from 6% to 52% and is usually of no clinical significance, with very minimal incidence of peripheral neuropathy [23]. In the present series the incidence of paravertebral leakage was 15% without any clinical manifestations.
Leakage of bone cement into the paravertebral veins may result in serious complications such as pulmonary or cerebral embolism, cardiac perforation, and death. High vascular vertebral pathologies, lower cement viscosity, and high injection force help leads to an increase in the intra-osseous pressure which facilitates the passage of fat and bone marrow into the venous circulation and the right heart [6,11]. Pulmonary cement embolism is not a common complication with vertebroplasty. It can be asymptomatic had been reported to occur in up to 4.6% of the patients [1,11]. Symptomatic cases present with chest pain, dyspnea and hypotension either immediate or delayed with a death risk [26]. Immediate ICU admission and medical management is required in symptomatic cases [26]. In the present series one patient experienced dyspnea after injection and was referred to the ICU and pulmonary embolism was diagnosed. There was no evidence of cement leakage during the injection and the patient showed a favorable recovery after treatment. Similarly, Syed et al. [26] described a similar complication following uinpedicular three levels PV in a patient who had no evidence of cement leakage and concluded that severe complications can also occur without cement leakages. Pulmonary embolism is not correlated to the number of vertebrae treated per session. This conclusion was reached by Benneker et al. [4] who performed a cadaveric study in 2008 and stated that reduction of the risk of cement embolization can be achieved by decreasing the force of injection as well as using a more viscous PMMA.
Pedicle fracture when passing the needle and posterior epidural leakage are also reported in the literature [20]. Murphy and Deramond [22] reported that complications associated with PV for osteoporotic fractures are less common than those in metastasis and hemangiomas. Leakage of bone cement into the epidural or foraminal space can range from asymptomatic cases to devastating motor complications up to paraplegia [7,17]. Paraplegia due to neural element compression related to bone cement is rare and occurs only in 0.4% of the cases [24]. Rib and transverse process fractures had also been reported to occur after PV [17]. Discitis, osteomyelitis and epidural abscess can result from infection following PV and may require surgery to remove the cement which acts as nidus for infection [34].
In the literature the incidence of new vertebral fracture adjacent or distant to fractured one after vertebroplasty ranged from 7 to 37% [14,27,29-31]. It is still unclear whether this is related to the natural history of the underlying disease or to the treatment. Lavanga et al. [16] stated the one session multilevel VP could be of help to prevent vertebral refractures even if there is not a true vertebral collapse. In the present study, we had a 10% incidence of new fractures. This was relatively consistent with what was reported by Kim et al. [14] who had a 7.9% incidence of new fractures as well as Mailli et al. [20] who had a 7.8% incidence of new fracture in their series of 77 osteoporotic patients. Also Uppin et al. [31] reported an incidence of 12.4% of new fractures after vertebroplasty. A higher incidence of new fractures was also reported by others [27,28,32] and ranged from 21 to 37%. The reason for lower incidence of refractures in the present study may be due to the multilevel injection as well as the small sample group and short term follow-up period. However, Lee et al. [18] in their analysis of risk factors causing new symptomatic vertebral compression fractures after PV for osteoporotic fractures found that aggressive bone mineral density and body mass index correction is more important than the vertebroplasty technique.
CONCLUSION
Multilevel PV for the treatment of osteoporotic fractures is a safe and successful procedure that can significantly reduce pain and improve patient’s condition without a significant morbidity. PV allows treatment of the already fractured vertebrae and reduces the incidence of refractures in osteoporosis. It is considered a cost effective procedure allowing a rapid restoration of patient mobility.
Notes
No potential conflict of interest relevant to this article was reported.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in this study.




