Glioblastoma Cellular Origin and the Firework Pattern of Cancer Genesis from the Subventricular Zone
Article information
Abstract
Glioblastoma (GBM) is a disease without any definite cure. Numerous approaches have been tested in efforts to conquer this brain disease, but patients invariably experience recurrence or develop resistance to treatment. New surgical tools, carefully chosen samples, and experimental methods are enabling discoveries at single-cell resolution. The present article reviews the cell-of-origin of isocitrate dehydrogenase (IDH)-wildtype GBM, beginning with the historical background for focusing on cellular origin and introducing the cancer genesis patterned on firework. The authors also review mutations associated with the senescence process in cells of the subventricular zone (SVZ), and biological validation of somatic mutations in a mouse SVZ model. Understanding GBM would facilitate research on the origin of other cancers and may catalyze the development of new management approaches or treatments against IDH-wildtype GBM.
INTRODUCTION
Isocitrate dehydrogenase (IDH)-wildtype glioblastoma (GBM) is a disease with a dismal prognosis, a distinction that has led to GBM being dubbed the “emperor of all cancers”. The disease progression is rapid, and even with standard therapy [53] and supra-total resection [52], the median survival of patients from diagnosis is approximately 14–20 months [53,63].
There are continuing efforts by researchers in diverse disciplines, including epidemiology and molecular biology, to identify the cause of GBM. Epidemiological research on GBM suggests numerous risk factors that should be taken into account, such as irradiation of the head [8,23,46,50,55] and the allergic history of patient [4]. Biological approaches for determining the cause of GBM include attempts to identify molecular signatures in patient samples (e.g., DNA mutations) [61], the use of in vivo mouse models [58], and investigation of progenitor cells of GBM [3,13,43].
This article reviews the proof-of-concept that GBM originates in the subventricular zone (SVZ) from the microscale perspective such as a molecular biology with a cellular level resolution. The first sections present a brief overview of the disease and recapitulate the importance of mutations in the development of GBM. The remaining sections describe the cell-of-origin of GBM and the cancer genesis patterned on firework from SVZ [43] and discuss a new hypothesis driving GBM research.
HISTORY : PROGRESS TOWARD THE STEM CELL ORIGIN OF GBM
There are plenty of discussions and arguments about the existence of the cell-of-origin from the early era of neuro-oncology whether the disease originates from embryonic cells or dedifferentiated cells [51]. The brain cancer model induced from the mutation of specific cells of brain is blurring the gap between those two sides [2] by leading an edge of this field with a subsequent drug discovery targeting GBM [58].
The cell-of-origin of GBM has been suspected to have characteristics of a stem cell, and this cell type has also been regarded as the source of tumor recurrence [22]. There have been many trials conducted to isolate such treatment-resistant insidious cells from the tumor tissues [3,34,41]. Those cells were labeled as tumorspheres [27,35,36] and their behaviors and characteristics have been validated from multiple sources [14,25,30,39,42,49,64]. As the patient-derived tumor cells are heterogeneous in the sample dimension (patient age, the size and location of the sample in the original tumor mass, sample control, purity of samples, or previous treatments), the mutation-induced tumor model has been accepted as another robust and predictable platform in the repeated experiments and clearly demonstrates the consequences of mutation [1,20,43] better than the orthotopic xenograft by primary tumorsphere [31,35,38,42].
An epiphany came from the experience of 5-aminolevulinic acid (5-ALA) fluorescence without any tumor cells in the tumor specimen by pathologic examinations [45]. During surgery to remove GBM, high fluorescence of 5-ALA was found in the walls of ventricles [45], which are non-coincidentally exposed after supra-total resection (or a so-called planned lobectomy) [52,53]. Moon et al. [45] described interesting patterns of 5-ALA fluorescence in the ventricular wall of brain tumor patients. What they found the most interesting was the ventricular walls with positive 5-ALA fluorescence without magnetic resonance imaging enhancement and no tumor cells in the pathology, a finding that perplexed surgeons [45].
Concurrence of 5-ALA positive tissues with no tumor cells in the ventricle sample [45] helped and guided us to find the origin of GBM in the in vivo model [43]. This 5-ALA glittering tumor negative samples from the ventricular wall or the SVZ has a pattern of mutations that has not been previously discovered. By comparing those mutation patterns, a novel way of understanding the disease could be revealed. The tumor-private mutations in the tumor tissue might facilitate the development of a new targeted therapy. In addition, the anatomical location of those cells would mature the Big Bang theory [7,60] into the Firework theory of subventricular abnormal cells [43].
ETIOLOGY
Epidemiologic studies have linked a number of pollutants to the incidence of GBM [48]. Ionizing radiation to the head at a younger age increases the risk of glioma and meningioma [8,23,46,50,55]. An allergic or atopic disease has been associated with a lower risk of GBM, and consistently with lower-grade glioma [4]. DNA mutations and its consequent syndromes increase the risk of acquiring GBM [32,48], such as Turcot syndrome (from mismatch repair-deficiency) [15], Li-Fraumeni syndrome (from the germline mutation of TP53) [37] and neurofibromatosis type 1 (from NF1 gene) [26,59].
However, there are additional unproven and controversial risk factors. One example is non-ionizing radiation from cellular phones. Studies have not shown a consistent increase in the risk of glioma [24]. Another example is Radon exposure which reported to be associated with the risk of glioma in a Danish cohort [9], but neither radon exposure nor background radiation was associated with glioma incidence in a cohort of British children [28]. Inconsistent results have been obtained with respect to the association of GBM development with exposure to air pollutants or particulate matter, owing in part to difficulties in measuring exposure history [5]. There is hardly any or no evidence linking insecticides, rubber processing, pesticides, farming, solvents, metal fumes, or jet engine manufacturing to the incidence of glioma [48,54].
IDENTIFICATION OF SOMATIC MUTATIONS IN SVZ STEM CELLS
Two senescence-associated mutational patterns were found in GBM : mutational signatures 1 and 5 [43]. These two types dominated the mutation profile of SVZ samples of the astrocytic ribbon (SVZ‡ in Fig. 1A, 33% [7.8 genes out of 23 total genes] and 45% [10.4 genes out of 23 total genes] for signature 1 and 5 respectively in the SVZ samples) [43].
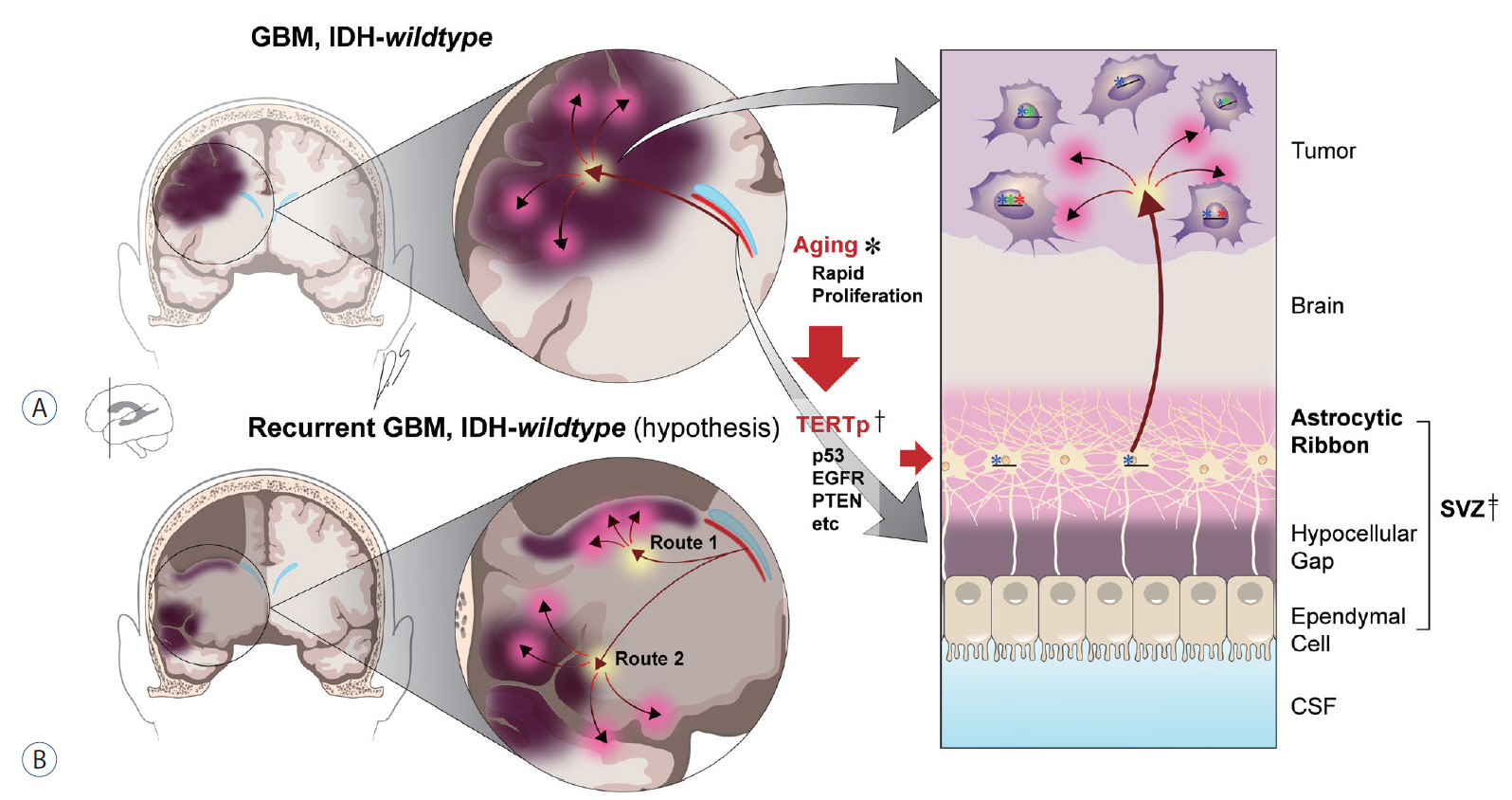
Cell-of-origin of IDH-wildtype GBM and GBM genesis mechanism. A : Illustration of IDH-wildtype GBM genesis from the SVZ43). Left : gross structure of the tumor in the brain. Red arrow (yellow background in the arrowhead) indicates migrating GBM origin cell from SVZ (red line under the sky blue lateral ventricle) to the hemisphere. Red arrow with a pink background in the arrowhead indicates the explosion (or evolutionary increase in heterogeneity) of tumor cells that creates the tumor bulk. Right : schematic overview of tumorigenesis from the SVZ to the cortex (below → top). Some portion of cells in the astrocytic band in the SVZ (‡) acquire mutations (illustrated by asterisks with blue in the SVZ; additional mutations are depicted in cells of the GBM in green and red asterisks) as an incidental consequence of senescence (*), with mutations in the TERT promoter serving as the possible initial step (†), followed by mutations in TP53, PTEN and EGFR , among others. B : Illustration of the hypothetical SVZ origin of IDH-wildtype GBM recurrence via route 1 or 2. Route 1 indicates same site recurrence, while route 2 indicates different site recurrence from the GBM cell-of-origin in the SVZ. GBM : glioblastoma, IDH : isocitrate dehydrogenase, TERTp : promoter of telomerase reverse transcriptase (Gene symbol : TERT), p53 : tumor protein p53 (Gene symbol : TP53), EGFR : epidermal growth factor receptor, PTEN : phosphatase and tensin homolog, CSF : cerebrospinal fluid, SVZ : subventricular zone.
The average number of genes for mutation signature 1 jumped up from 7.8 genes (SVZ) to 81.9 genes in tumor samples of the Severance GBM cohort (10.5 fold increase) [43]. While the fold change of average number of genes that contribute to mutation signature 5 (fold 0.71, calculated from 10.4 genes of SVZ to 7.4 genes of the tumor) remains relatively stable as that of other signatures (fold 1.18, calculated from 4.8 genes of SVZ to 5.7 genes in the tumor) [43]. These findings suggest that GBM arises from the senescence process, especially if we focus on the number of genes of mutational signature 1 and 5 from SVZ to tumor, or alternatively from other causes that are associated with age.
DIRECTION OF CLONAL EVOLUTION
Accumulation of mutations in both the stem cells and the tumor region raises questions regarding the origin of the mutations. Are mutations in both tumors and SVZ related? Are they coincidental? Do mutations in the SVZ reflect contamination from the tumor? To deconvolute this complex pattern of mutations, the authors compared shared mutations (i.e., those in common to both the SVZ and tumor) and tumor-exclusive mutations (i.e., those not found in the SVZ) [43]. There were several shared mutations in the tumor-free SVZ and GBM bulk tumor, including those in the TERT promoter [43]. An analysis of variant allele frequencies (VAFs) and single-cell sequencing results revealed a unidirectional pattern from SVZ to bulk tumor. A two-dimensional VAF plot in a previous report [43] showed unique patterns of shared mutations between the tumor and the SVZ, with low-level driver mutations being located near the y-axis or tumor-axis. However, the tumor-contaminated SVZ-tumor pair showed more genes that are spread along the identity line as opposed to the uncontaminated SVZ-tumor pair [43] (Fig. 1A).
Single-cell sequencing of the DNA of bulk tumors also validated the result showing directionality from the SVZ to the tumor [43]. Twenty-four clones were derived from one IDH-wildtype GBM patient (GBM245) [43]. The genes on the table of Lee et al. [43] are subset of those genes of directionality. This surprising pattern of mutation was later confirmed from the intraventricular injection of plasmids to induce mutation in the SVZ [16,43].
PROGRESSION OF MUTATIONS IN GBM FROM THE SVZ
Mutations oftentimes start with TERT promoter mutations in cells of the astrocytic ribbon in SVZ under the ventricle (TERTp† in Fig. 1A). With additional mutations in oncogenes, the mutated cells from SVZ migrate to the cortex and manifest as the GBM (Fig. 1A, right) [43]. The authors searched for mutations in triple-matched samples of tumor-free SVZ, GBM tumor, and normal brain cortex (or blood) [43]. The authors have hypothesized that the SVZ, which includes neural stem cells, is suspected of being the origin of tumors [43]; mutations then progress from the SVZ to the tumor mass. In addition to this directionality, the number of mutations increases dramatically after the cell-of-origin reaches the cortex. As described in the previous study of Lee et al. [43], about 20 different somatic mutations of SVZ that contribute to the mutational patterns increased to around 100 mutations in the tumor. Furthermore, mutational signature 1 dominated most mutations in tumors, whereas mutational signature 5 was preserved at about the same level between the SVZ and the tumor [43]. This pattern is slightly different version of the Big Bang concept and, considering the anatomic location of the progression, the authors refer to it as a “Firework” pattern (Fig. 2).
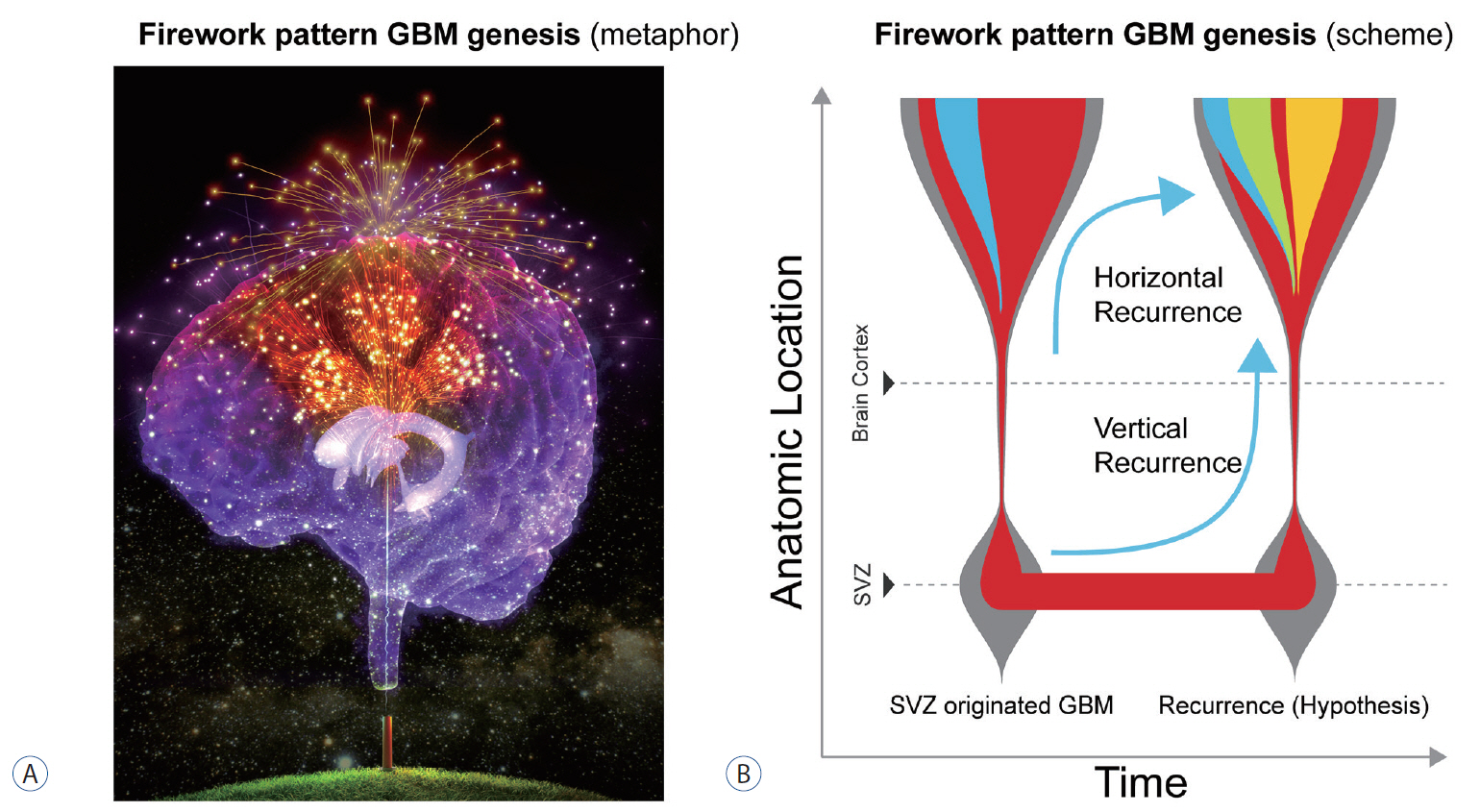
Firework pattern of IDH-wildtype GBM genesis. A : Artistic illustration of IDH-wildtype GBM originating from the SVZ (colored in gold). Each firework trail corresponds to a different cancer clone. In this metaphorical depiction, the SVZ is represented as a cannon on the ground, denoting the starting point of GBM genesis. B : Conceptual illustration of the time line of the genesis of the firework pattern of IDH-wildtype GBM. Horizontal recurrence (or classical model) : GBM recurs from SVZ (red) → tumor (blue) → recurrence (green), vertical recurrence (hypothetical model) : primary GBM originates from SVZ (red) → tumor (blue), and it recurs from SVZ (red) → recurrent tumor (orange). GBM : glioblastoma, SVZ : subventricular zone, IDH : isocitrate dehydrogenase.
BIOLOGICAL VALIDATION
Deep under the ependymal layer of the SVZ is a ribbon-like feature called the astrocytic ribbon [57] (SVZ‡ in Fig. 1A). This area is populated by neural stem cells in both mouse and human brains [65]. Using molecular techniques described below, we introduced three types of mutations into the population of cells in the SVZ. These mutation-bearing cells migrated to the cortex, ultimately forming high-grade gliomas [43] (Fig. 1A), presenting with firework-like GBM genesis (Fig. 2A).
A mass of GBM is created in the cortex by the mutation in the SVZ cells [43]. Crossbred mice (postnatal day 2–3 pups) carrying LSL-tdTomato and LSL-EGFRviii [71] were used for these experiments [43]. A Cre-containing CRISPR/Cas9 plasmid was electroporated into one side of the ventricle in the brain to knock out Pten and Trp53 (mouse analog of human TP53) genes using single guide RNA (sgRNA) for these genes. After injection into the ventricular space with a sharp needle, the electroporation device was applied to deliver the plasmid into the SVZ [43]. In the presence of the mutations, brain tumors developed in 90% of mice (9/10), with 67% of tumors developing in a region distant from the mutation-arising SVZ, and these mice showed a median survival of around 20 weeks [43].
Tumorigenesis, or Cancer genesis from the triple mutations marks the important step in the research of GBM [61]. By this finding [43], more subtle nature of IDH-wildtype GBM can be revealed in the subsequent researches.
HYPOTHESIS : RECURRENCE OF GBM FROM THE SVZ
One of the next steps in the research of IDH-wildtype GBM should be related with the recurrence pattern of GBM (The hypothesis on the recurrence of GBM). Careful selection of samples without tumor in the SVZ is the beginning point of this research. Recent article by Watts group [61] is one example of selecting the tumor-invaded SVZ (median tumor content 22.1%, rather than tumor-free SVZ) by the IDH-wildtype GBM and its results and implications should be carefully contemplated in the research of vertical recurrence from the cell of SVZ (Fig. 2B).
GBM recurs after irradiation of tumor margins at varying depth [69]. Although irradiating the SVZ appears to have a prognostic benefit for GBM patients [29,47], it does not cure these patients or prevent exacerbation of symptoms. There are at least two scenarios for recurrence of IDH-wildtype GBM. In the first case, GBM-initiating cells already present in the parenchyma of the brain restart the tumor after a dormant period (Fig. 2B, horizontal recurrence). In the second, dormant cells in the SVZ re-migrate to the tumor bed area, where they create the recurrent tumor (Fig. 1B, the only hypothesis in this article; Fig. 2B, vertical recurrence). We hope to resolve the second phenomenon using tumor-free SVZ samples from GBM patients.
FUTURE : DEVELOPMENT OF THERAPEUTICS TARGETING THE SVZ
Many previous attempts to treat GBM have failed and the list harbors 5-f luorouracil and methotrexate [10]. A breakthrough in treating GBM came with the application of alkylating agents [18,62,67]. Nitrosoureas proved the most effective at the time, and it became the most commonly used drugs for the management of brain tumors, including GBM [56,70]. Their antitumor effects were widely accepted and even led to the development of an implantable chemotherapeutic system impregnated with the nitrosourea derivative carmustine [11].
However, these nitrosoureas had toxicity issues, and temozolomide subsequently gained popularity because of its limited adverse effects compared with earlier alkylating agents [6]. But even temozolomide proved to be ineffective in treatment of recurrent GBM similarly to other alkylating agents [33,44,66]. And the therapeutic efficacy of other novel options is limited in Bevacizumab [19,21,68], IDH-targeting drugs [12] and immunotherapy [17,40].
After the repeated efforts to treat the tumor itself, the origin of GBM emerged as the next generation target. Ultimately, in vitro and in vivo models will accelerate the screening for drugs that can preemptively target the initial process of cancer genesis with firework pattern from SVZ.
CONCLUSION
Mutated neural stem cells, that are present in the astrocytic ribbon in the SVZ, may migrate to the brain cortex and generate IDH-wildtype GBM with/or without any molecular or environmental cue [43]. While this concept has been demonstrated scientifically, elucidating the detailed mechanism underlying the GBM genesis process will require additional investigation. Our ongoing research efforts would focus on identifying the cell-of-origin to develop a potential treatment option for IDH–wildtype GBM, the “Emperor of cancer”.
Notes
No potential conflict of interest relevant to this article was reported.
INFORMED CONSENT
This type of study does not require informed consent.
AUTHOR CONTRIBUTIONS
Conceptualization : SJY, JHM, EHK, JHC, JHL, SGK
Data curation : SJY, SGK
Funding acquisition : SGK
Methodology : SJY, HJK, JHL, RJC, JKS, JHL, SGK
Project administration : SGK
Visualization : SJY, DSJ, SGK
Writing - original draft : SJY, SGK
Writing - review & editing : SJY, JP, HJK, JHL, EJ, RJC, JKS, JHM, EHK, JHC, JHL, SGK
Acknowledgements
This work was supported by grants from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI17C2586), the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (NRF-2019R1A2C3004155), and the NRF grant funded by the MSIP : Ministry of Science, ICT and Future Planning, Republic of Korea (NRF-2017M2A2A7A01071).
