Reliability of Early Ambulation after Intradural Spine Surgery : Risk Factors and a Preventive Method for Cerebrospinal Fluid Leak Related Complications
Article information
Abstract
Objective
Cerebrospinal fluid leakage related complications (CLC) occasionally occur after intradural spinal surgery. We sought to investigate the effectiveness of early ambulation after intradural spinal surgery and analyze the risk factors for CLC.
Methods
For this retrospective cohort study, we enrolled 314 patients who underwent intradural spinal surgery at a single institution. The early group contained 79 patients who started ambulation after 1 day of bedrest without position restrictions, while the late group consisted of 235 patients who started ambulation after at least 3 days of bed rest and were limited to the prone position after surgery. In the early group, Prolene 6–0 was used as the dura suture material, while black silk 5–0 was used as the dura suture material in the late group.
Results
The overall incidence rate of CLC was 10.8%. Significant differences between the early and late groups were identified in the rate of CLC (2.5% vs. 13.6%), surgical repair required (1.3% vs. 7.7%), and length of hospital stay (2.99 vs. 9.29 days) (p<0.05). Logistic regression analysis revealed that CLC was associated with practices specific to the late group (p=0.011) and the revision surgery (p=0.022).
Conclusion
Using Prolene 6–0 as a dura suture material for intradural spinal surgery resulted in lower CLC rates compared to black silk 5–0 sutures despite a shorter bed rest period. Our findings revealed that suture - needle ratio related to dura defect was the most critical factor for CLC. One-day ambulation after primary dura closure using Prolene 6–0 sutures appears to be a cost-effective and safe strategy for intradural spinal surgery.
INTRODUCTION
Cerebrospinal fluid (CSF) leakage related complications (CLC) occur after intradural spinal surgery at an incidence rate that ranges from 1.5% to 13% [7,14,15,23]. Postoperative CSF leakage often requires secondary interventions, and CLC after spinal surgery constitute a major source of patient morbidity and economic burden [27]. In some cases, wound healing is delayed due to pseudomeningocele or durocutaneous fistulas, which may require re-operation. CLC may also extend the duration of the inpatient postoperative care period. Prolonged bed rest decreases the CSF pressure at the site of the durotomy, thereby reducing the flow of CSF through the dural defect and potentially reducing these complications [2,3,5]. However, there is insufficient evidence from which to form a consensus among spinal surgeons. Moreover, complications of bed rest in the prone position, such as thromboembolism, should not be overlooked [4,22].
Although there are a number of risk factors that may contribute to the occurrence of CLC, they are not well defined [2]. A watertight closure and proper suture material are critical factors for an effective intraoperative management strategy [6]. In one large scale study, a 98.2% success rate was obtained using 5–0 silk sutures for a dura tear [16]. In contrast, 6–0 Prolene demonstrated the best results for watertight closure in recent experimental research on the hydrostatic strength of dural repairs using various suture sizes [1,4].
So far, there have been no direct comparative clinical studies of postoperative bed rest periods and respective suture materials in a single-center setting. In the present study, we evaluated the efficacy of postoperative 1-day ambulation compared to a more extended bed rest period as a routine postoperative care protocol following intradural spinal surgery. We furthermore investigated the risk factors for the occurrence of CLC.
MATERIALS AND METHODS
This study received approval from the Institutional Review Board of Asan Medical Center (IRB approval No. AMC IRB 2020-0711).
Study design
We conducted a retrospective cohort study to evaluate the effects of dura suture material and postoperative bed rest after intradural spinal surgery. The patients were divided into an early ambulation (early) group, comprising patients who start ambulation on the first day after surgery, and a late ambulation (late) group, comprising patients who start ambulation on the third day after surgery or later, depending on wound status. In the early group, polypropylene 6–0 Prolene sutures with a 9.3 mm 3/8c round bodied needle (Ethicon Inc., Somerville, NJ, USA) were used as the dural suture material. Patients who had surgery between March 2018 and December 2019 were included in this group. In the late group, braided non-absorbable 5–0 black silk sutures with a 10 mm 3/8c round bodied needle (Ailee, Busan, Korea) were used as the dural suture material. Patients who had surgery between January 2012 and December 2018 were included in this group. Clinical characteristics, pathology, surgical outcomes, CLC, and surgical repair rate were compared between the groups.
Study participants and data collection
We reviewed all patients who underwent intradural spinal surgery in Asan Medical Center between January 2012 and December 2019. The inclusion criteria were as follows : 1) patients with intradural tumors or vascular malformations; 2) patients who underwent posterior midline approach and durotomy after laminectomy or laminotomy; 3) patients in whom primary dura closure was performed; and 4) those with a follow-up period ≥6 months. Patients who had surgery due to trauma or infectious causes, extradural lesions, or who had undergone additional anterior surgical procedures, duroplasty, or incomplete dura closure, as well as patients who had simple surgical site infections without CSF leaks were excluded. Operations on patients included in the early group were performed by a junior surgeon (name-blinded) and operations on patients in the late group were performed by a senior surgeon (name-blinded). Patients attended a postoperative outpatient clinic at 1 month (or earlier if necessary) and 6 months post-surgery for assessment of clinical symptoms and wound status follow-up.
Clinical data were obtained from our institutional electronic medical records. Patient age, sex, body mass index, smoking, diabetes mellitus, antiplatelet or anticoagulation agent status, pathologic diagnosis, location, revisional skin incision, and durotomy associated with a previous scar or first surgery were recorded. To evaluate the surgical outcomes and complications after surgery, we also recorded data on the performance and level of laminectomy or laminotomy, postoperative hospital days, bedrest days, and McCormick grade.
Surgical technique and postoperative management
Dura closures were all performed using a running locked suture technique (Fig. 1). The throws were approximately 1 to 2 mm apart and these were tied with a surgeon’s knot at each end under microscopic magnification using microsurgical instruments (Castroviejo needle holders, Gerald tissue forceps). Following completion of the dura closure, a Valsalva maneuver was used to check for CSF leakage, and the fibrin sealant patch (TachoSil, Baxter, CA, USA) was applied. There was an intentional effort to perform laminotomy to the maximum possible extent on patients in the early group. Laminoplasties were performed using Lorenz Plating System Neuro Implants (Biomet, Jacksonville, FL, USA) or Centerpiece Hinge plates (Medtronic, Memphis, TN, USA). No patient in this study received the placement of a subfascial wound drain. The muscle fascia was closed as tightly as possible using 1–0 Vicryl sutures (Ethicon Inc.).

Intraoperative microscopic image of dura closure performed using running locked suture technique. Prolene 6–0 suture was used for the early group patients (A); black silk 5–0 suture was used for the late group patients (B).
Early group patients routinely started walking on the first day after surgery, and tolerable ambulation was maintained when symptoms such as hypotensive headache occurred. Late group patients routinely stayed on bedrest in the prone position for 3 days, with daily monitoring of the condition of the wound. In cases where fluid collection or wound bulging was observed, the bed rest period was prolonged. In the late group only, a lumbar drain was considered when CSF leakage occurred or when a definite pseudomeningocele was present.
Statistical analysis
Variables between the early and late groups were compared using an independent t-test, Mann-Whitney U test, and chisquared test. To adjust for confounding variables, a multivariate logistic regression analysis was performed on the risk factors associated with CLC that were identified as significant in a simple logistic regression analysis. The odds ratios (ORs) of the risk factors were obtained using the chi-squared test. All data management and statistical analyses were performed using SPSS software (version 20.0; IBM Corporation, Armonk, NY, USA). A p-value <0.05 was considered significant.
RESULTS
Patient demographics and clinical characteristics
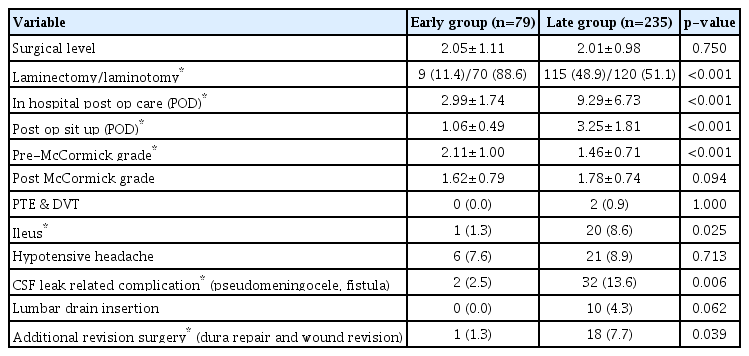
There were no differences in sex, smoking, diabetes mellitus, use of anticoagulants or antiplatelet agents, revision surgeries (when approaching through the previous wound), or distribution of surgical location. The average age was significantly higher in the early group (55.62 vs. 50.70 years, p=0.013), as was the rate of intramedullary lesions (22.8% vs. 12.3%, p=0.024). Intradural lesions consisted of tumors and vascular malformations (cavernoma, arteriovenous fistula), and did not differ significantly between the two groups. Schwannomas (55.4%) were the most common, followed by meningiomas and ependymomas (Table 1).
Surgical outcomes and complication
The range of surgical levels did not differ between the two groups, and laminoplastic laminotomy was performed at a higher rate in the early group patients than in the late group patients (88.6% vs. 51.1%, p<0.001). Patients in the early group were discharged significantly faster (postoperative 2.99 days vs. 9.29 days, p<0.001). They also achieved ambulation earlier after sitting up (postoperative 1.06 days vs. 3.25 days, p<0.001) (Fig. 2).

Early group patients were discharged significantly faster (postoperative 2.99 days vs. 9.29 days, p<0.001), they started ambulation earlier after sit up (postoperative 1.06 days vs. 3.25 days, p<0.001). Despite the shorter duration of the early group’s hospital stay, the overall CLC rate was low. CLC : cerebrospinal fluid leakage related complications.
Since most of the diseases included in this study were spinal cord tumors, we utilized the McCormick grade for the comparison of neurologic outcomes. The early group demonstrated a higher grade prior to surgery (2.11 vs. 1.46, p<0.001), but there was no significant difference between the two groups after surgery (1.62 vs. 1.78, p=0.094).
Ileus is a complication that may occur in relation to bed rest after surgery. In the early group, the rate of ileus was significantly lower compared to the late group (1.3% vs. 8.6%, p=0.025). The rate of fatal complications such as pulmonary thromboembolism and deep vein thrombosis did not differ significantly between groups. There were two cases in the late group and no cases in the early group. There was also no difference in the frequency of hypotensive headache, one of the symptoms of CSF leakage (7.6% vs. 8.9%, p=0.713). CLC including symptomatic pseudomeningocele, durocutaneous fistula, and wound dehiscence with oozing were significantly reduced in the early group (2.5% vs. 13.6%, p=0.006). In the late group, 10 patients had a lumbar drain after surgery, while there were no cases in the early group that required a lumbar drain. Despite meticulous conservative treatment and close observation, one patient (1.3%) in the early group underwent additional repair surgery due to worsening progression, compared to 18 patients (7.7%) in the late group, a difference that was statistically significant (p=0.039) (Table 2).
Risk factors of CLC
In simple logistic regression analysis, an increased incidence of postoperative CLC was significantly associated with the late group (p=0.015), as well as day of postoperative sit-up (p<0.001), and re-operation (p=0.039) (Table 3). Assignment to the late group (p=0.011) and revision surgery (p=0.022) remained significant risk factors for postoperative CLC following multivariate analysis (Table 4).
DISCUSSION
This study sought to investigate the efficacy of routine 1-day ambulation after intradural spinal surgery. We compared two groups that differed in the suture material used for dural closure and in strategies for postoperative care, according to the policies of each surgeon.
Patient positioning after a durotomy remains controversial. Conventional wisdom suggests that positioning the patient in the prone position after intradural surgery will decrease the CSF pressure at the site of the durotomy, thereby decreasing the flow of CSF through the dural defect [2]. In patients with symptoms such as postural headache, several studies have reported that CLC could be prevented by limiting the patient to the prone position for several days after lumbar durotomy [10,18,20,21]. However, there have also been reports that a shortened or even absent bed rest period is not associated with inferior outcomes [13,19,22]. Prolonged bed rest causes multiple complications and increases medical expenses [4]. Therefore, early ambulation could prove beneficial; however, more evidence is required. In the cohort presented here, two of the late group patients had pulmonary thromboembolism and deep vein thrombosis. In addition, the incidence of ileus was significantly higher in the late group. In contrast, early group patients had significantly shorter hospital stays after surgery. Therefore, our findings demonstrate that early ambulation after primary dura closure with Prolene 6–0 sutures could result in reduced health care costs (Fig. 2).
In the present study, the overall incidence of CLC after intradural tumor surgery was 8.39%. While we had expected that the CLC rate of the early group would not be inferior to the late group, the results showed that the late group had a significantly higher CLC rate (early : 2.5% vs. late : 13.6%, p=0.006). The most likely reason for these findings was the difference in dura suture material between groups. Based on the experimental evidence, the choice of suture material could result in a disparity in closure strength [1,4,9]. A previous study using a calf spine model compared braided nylon (Surgilon 5–0; Ethicon Inc.) and Prolene 6–0. Prolene 6–0 was found to produce a more watertight closure at higher hydrostatic pressures [4]. In the case series presented here, we demonstrated similar outcomes for the clinical utility of Prolene 6–0. Furthermore, the needle diameter for black silk 5–0 was thicker than that for Prolene 6–0 (0.30 vs. 0.25 mm), resulting in a relatively more substantial dura defect along the hole where the needle passed. All dura closures in both groups were performed using a running locked suture technique. We did not investigate the difference between suture techniques, as previous studies have already demonstrated no significant differences in CSF leakage between interrupted and continuous running locked suture techniques [4,9].
A widely accepted management option for CLC occurrence is surgical repair either by meticulous direct primary closure of the dura or through augmented closure by means of fat, muscle tissue, or a fascial graft [11,21]. In our study, CLC was resolved through revision surgery in 19 cases (6.1%, 19/314). In the early group, only one (1.3%, 1/79) surgical repair was performed. Guerin et al. [11] reported that CSF leaks can be managed by observation alone while on bed rest or by the placement of a shunt either during the index procedure or in the postoperative period. Consistent with this, the late group patients in our study were routinely ordered to maintain bed rest in the prone position for 3 days or more, and a lumbar drain was inserted during the observation period when pseudomeningocele or high CLC risk was present, based on the judgment of the senior surgeon. Ten patients temporarily maintained CSF shunts with a lumbar drain, and eight of them healed without revision surgery.
In univariate logistic regression analysis for CLC risk factors, inclusion in the late group, revision surgery, and day of postoperative sit-up were all significant. The results showed that the CLC rate increased in proportion to the length of time between surgery and the day of postoperative sit-up. These findings deviated from that which we expected based on causative influences. It is possible that these results are due to the more extended bed rest period experienced by patients in the late group, as well as the postoperative treatment protocol that further prolongs bed rest if empirically assessed as a high risk for CLC. Thus, the postoperative sit-up day factor was excluded in multivariate analysis. Following multivariate logistic regression analysis, inclusion in the late group (primary dura closure using black silk 5–0 suture) and revision surgery (re-exposed through scar) continued to be significant risk factors for CLC. In cases of CSF leakage from the durotomy site, the muscle fascia layer becomes a barrier and prevents CLC. Therefore, a revision surgery patient has delayed wound healing compared to a primary surgery patient. Revision surgery also results in a large dead space due to granular scar tissues, which may have influenced these results. The infection rate might also be influenced for the same reason. In a large scale study of surgical site infections, revision surgery was associated with a significantly higher infection rate than primary surgery [24].
The surgical fellow who performed the dural closure (p=0.052) showed numerical differences in CLC rates, although this was not statistically significant (0.05<p<0.10). The proficiency of the surgical fellows who actually close the dura could affect the CLC rate. Differences in outcomes depending on who performed the dural closure suggest the importance of careful manipulation when performing this procedure. The precise technique required to attain a watertight closing of the muscle fascia while minimizing dead space is also essential.
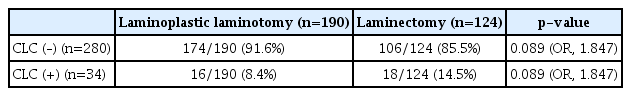
In our result, laminectomy did not have a statistically significant effect on the CLC rate. CLC occurred in 16 of 190 patients (8.4%) who underwent laminoplastic laminotomy, and in 18 of 124 patients (14.5%) with laminectomy by subgroup head to head analysis using the chi-square test (OR, 1.847; p=0.089; Table 5). However, laminoplasty after dura closure could reduce CLC rates compared to laminectomy, as demonstrated in a previous meta-analysis comparing the two methods [26]. A possible explanation for this is that the lamina between the dura and the muscle becomes a barrier and reduces dead space [17]. Studies in which minimally invasive surgeries were conducted for intradural lesions reported low rates of CLC [8,28]. In one such study, the limited soft-tissue exposure and a relative absence of dead space led to a relative increase in epidural pressure, “tamponading” the epidural space and preventing CSF leakage [28].
In the patient demographics, the mean age was significantly different between the two groups. Advanced age is a risk factor for unintended durotomy that occurs during degenerative spine surgery [25], and the rate of CLC after incidental dural tear increases with age [11]. However, in another study of the cervical spine, post-operative CSF leak and patient age were irrelevant [12]. Furthermore, it is unclear whether age is a risk factor for CLC after intended durotomy procedures such as intradural tumor surgery [2]. Also, in the present study, patient age was not a significant risk factor by logistic regression analysis.
The present study has several limitations. It was a retrospective study, which prevented randomization of the clinical series of patients. In patient demographics, mean age of both groups were different. Additionally, we investigated two different factors between the groups : suture material and postoperative care strategy with bed rest period. Thus, we were unable to assess whether postoperative 1-day ambulation is safe irrespective of suture material. However, the findings described here are meaningful because they demonstrate the efficacy of early ambulation in the context of Prolene 6–0 sutures.
CONCLUSION
Our findings revealed that intraoperative manipulation had a more significant effect than postoperative management for the prevention of CLC, and that dura suture - needle ratio related to defect along the hole where the needle passed was the most important factor. Dura closure with Prolene 6–0 was associated with significantly lower CLC rates compared to black silk 5–0 with an extended bed rest period, even if early ambulation was begun on the first day after surgery. Postoperative 1-day ambulation combined with Prolene 6–0 sutures appears to be a cost-effective and safe management protocol for intradural spinal surgery.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
INFORMED CONSENT
This type of study does not require informed consent.
AUTHOR CONTRIBUTIONS
Conceptualization : SL, JHP
Data curation : SCR, KTK
Formal analysis : YSL, DCC
Funding acquisition : JHP
Methodology : DCC, KTK
Project administration : SCR, JHP
Visualization : DCC, YSL
Writing - original draft : SL
Writing - review & editing : SL, SCR, JHP