Clinical Characteristics and Current Managements for Patients with Chronic Subdural Hematoma : A Retrospective Multicenter Pilot Study in the Republic of Korea
Article information
Abstract
Objective
Chronic subdural hematoma (CSDH) is a common disease in neurosurgical departments, but optimal perioperative management guidelines have not yet been established. We aimed to assess the current clinical management and outcomes for CSDH patients and identify prognostic factors for CSDH recurrence.
Methods
We enrolled a total of 293 consecutive patients with CSDH who underwent burr hole craniostomy at seven institutions in 2018. Clinical and surgery-related characteristics and surgical outcomes were analyzed. The cohort included 208 men and 85 women.
Results
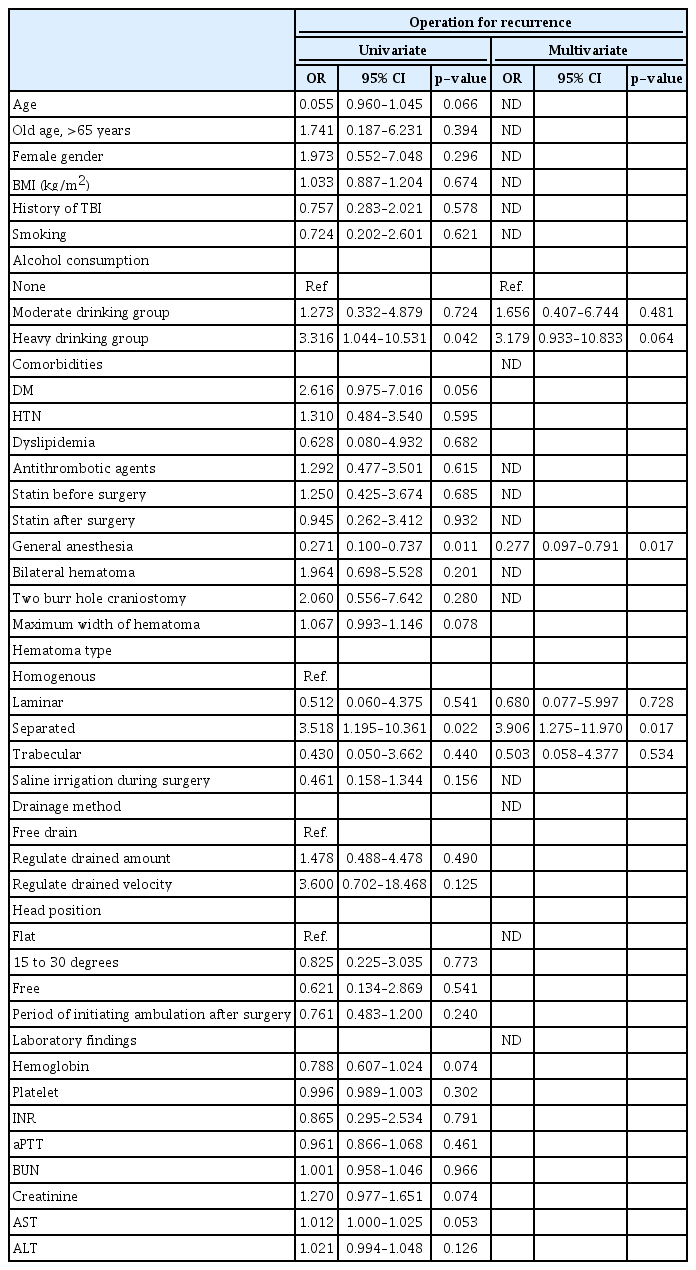
The median patient age was 75 years. Antithrombotic agents were prescribed to 105 patients. History of head trauma was identified in 59% of patients. Two hundred twenty-seven of 293 patients (77.5%) had unilateral hematoma and 46.1% had a homogenous hematoma type. About 70% of patients underwent surgery under general anesthesia, and 74.7% underwent a single burr hole craniostomy surgery. Recurrence requiring surgery was observed in 17 of 293 patients (5.8%), with a median of 32 days to recurrence. The postoperative complication rate was 4.1%. In multivariate analysis, factors associated with CSDH recurrence were separated hematoma type (odds ratio, 3.906; p=0.017) and patient who underwent surgery under general anesthesia had less recurrence (odds ratio, 0.277; p=0.017).
Conclusion
This is the first retrospective multicenter generalized cohort pilot study in the Republic of Korea as a first step towards the development of Korean clinical practice guidelines for CSDH. The type of hematoma and anesthesia was associated with CSDH recurrence. Although the detailed surgical method differs depending on the institution, the surgical treatment of CSDH was effective. Further studies may establish appropriate management guidelines to minimize CSDH recurrence.
INTRODUCTION
Chronic subdural hematoma (CSDH) is an encapsulated collection of blood and blood degradation products layered between the dura mater and the arachnoid membrane [13]. CSDH is a common disease, especially in elderly patients in a neurosurgical department [45]. The reported incidence of CSDH varies from 8.8 to 79.4 per 100000 persons per year, with an increasing trend in elderly patients [2,4,5,37]. The incidence of CSDH increases as the population ages [32,37]. Neifert et al. [32] reported that the incidence of CSDH in the United States is expected to increase significantly from 2020 to 2040, with the majority of cases occurring in elderly patients. Rauhala et al. [37] reported the epidemiological findings of a Finnish CSDH cohort and demonstrated that the incidence of CSDH has nearly tripled in the population aged 80 years or older since 1990. The global population is growing older, and the population aged 80 years or over is projected to triple by 2050 [42] therefore, the healthcare burden related to CSDH will increase in neurosurgical practice.
Symptoms in patients with CSDH include headache, cognitive impairment, gait disturbance, or motor weakness. Although spontaneous resolution can occur, surgical treatment is indicated for symptomatic patients with CSDH [8]. Surgical treatments, including burr hole craniostomy, twist drill craniostomy, and craniotomy, for CSDH remain safe and effective treatment modalities for symptomatic patients [15,23,45]. However, CSDH has a relatively high rate of recurrence, ranging from 10% to 20% for patients treated with surgery [3,23]. Various pre- and post-operative management methods, such as patient positioning or corticosteroid, antiplatelet, and anticoagulant use, may also be associated with outcomes of CSDH [23]. However, to date, there is little level I evidence describing optimal perioperative management strategies [23]. Therefore, it is difficult to properly suggest appropriate treatment and follow-up management strategies.
Well-established clinical practice guidelines can contribute to better management strategies for patients with CSDH. The Neurotrauma Clinical Practice Guidelines Committee of the Korean Neurotraumatology Society was organized to prepare treatment guidelines for the management of patients with traumatic brain injuries. Therefore, the present study aimed to present the current clinical, treatment, and outcome characteristics of CSDH patient in the Republic of Korea through the first multicenter study of a generalized CSDH cohort to provide appropriate CSDH treatment guidelines in near future.
MATERIALS AND METHODS
Participants and study settings
This study was approved by the Institutional Review Boards of seven institutions (Soonchunhyang University Cheonan Hospital, IRB No. SCHCA 2020-06-020; Yeungnam University Hospital, IRB No. 2020-06-046; St. Vincent's Hospital, IRB No. VC20RADI0130; Chungnam National University Hospital, IRB No. CNUH 2020-07-066; Dongsan Medical Center, IRB No. 2020-06-062-003; Uijeongbu St. Mary's Hospital, IRB No. UC20RIDI0102; Korea University Guro Hospital, IRB No. 2020GR0319). We conducted this retrospective, multicenter pilot study to assess the CSDH situation in the Republic of Korea. We retrospectively reviewed medical records and radiological imaging studies of adult patients (>18 years) who were diagnosed with CSDH and surgically treated between January 1, 2018 and December 31, 2018 at one of the seven participating institutions. A total of 299 patients underwent surgical treatment for CSDH. The following patients were excluded : five patients with no postoperative imaging studies and one patient who was treated with craniotomy for CSDH. A total of 293 patients were enrolled in this study, and all of them underwent burr hole trephination and drainage. Surgical techniques and postoperative management were performed according to the local policy.
Characteristics variables
Data were collected from the electronic medical records at each medical center. The data were investigated by dividing them into basic medical information and surgical information.
Basic medical information included patients demographics, symptoms and signs, preoperative mobility level, preoperative Markwalder’s grade [27] and Glasgow coma scale score, history of head trauma, period until admission from trauma, degree of alcohol consumption, history of smoking, comorbidities, past history of brain lesions, preoperative medication, and laboratory findings. Antithrombotic agents included two anticoagulant agents (warfarin, novel oral anticoagulants) and three antiplatelet agents (aspirin, clopidogrel, and cilostazol). Moreover, antilipidemic and anti-hypertensive agents were included in this study. Patients were categorized into four groups based on preoperative mobility : an independent group that could walk freely, a group that needed assistance while walking, a wheelchair group, and a bedridden group. The patients were categorized according to their degree of alcohol consumption into heavy drinking, moderate drinking, and no drinking groups. Heavy drinking group included those who met the criterion of consuming more than 4 drinks on any given day for men or more than 3 drinks for women, according to the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The appropriate drinking group was defined as the drinking group that did not meet the criteria for the heavy drinking group.
Findings of radiological diagnostic tools, including computed tomography (CT) and magnetic resonance imaging (MRI), were reviewed. Based on preoperative CT and/or MRI, radiological characteristics, such as location and maximum width of hematoma and type of CSDH were reviewed. Hematomas were classified into four types based on radiologic images, as previously described by Nakaguchi et al. [31] : homogenous, laminar, separated, and trabecular (Fig. 1).

Computed tomography demonstrating four types of chronic subdural hematoma. A : Homogeneous type. B : Laminar type. The arrowheads indicate the high-density laminar structure running along the inner membrane. C : Separated type. The arrow indicates a thick component of the liquefied hematoma. D : Trabecular type. The arrow indicates multiple septations created by fibrosis.
Surgical information included the method of anesthesia (local or general), location of the burr hole, number of burr holes, saline irrigation, method of drainage, usage of urokinase or tissue plasminogen activator (tPA), angle of bed maintained during drainage (flat, 15 to 30 degrees, or free), and use of medication after surgery (tranexamic acid, statin, or steroid). The method of drainage was classified as “free drain” or ‘‘amount-regulation’’ (a method of re-drainage after checking the total amount of drainage and stopping for 2 hours) or ‘‘velocity-regulating’’ (a method of adjustment of the amount of drainage per hour). In addition, the drainage maintenance period, hospitalization period, and recurrence period after the first operation were investigated.
The postoperative outcome was assessed using the Glasgow outcome scale [17]. Complications related to surgery were defined as newly developed problems occurring after surgical treatment for CSDH. Recurrence was defined as the re-accumulation of hematoma on the ipsilateral side, for which surgical treatment was required within 6 months of the initial surgery.
Statistical analysis
The results were analyzed using IBM SPSS Statistics software (version 25.0; IBM Corp., Armonk, NY, USA). Descriptive statistics, such as frequency (n), percentage, median, mean, and range were used to describe variables and subgroup characteristics. When evaluating the univariate association with CSDH recurrence, a Pearson’s chi-square test, Fisher’s exact test, and linear-by-linear association analysis were used for categorical variables, and a Student’s t-test was used for non-categorical variables. Logistic regression analysis was performed to analyze the independent associations between recurrence and contributing factors. Variables with p<0.05 in the univariate analysis were included in a multivariable analysis. Values of p<0.05 were accepted as statistically significant.
RESULTS
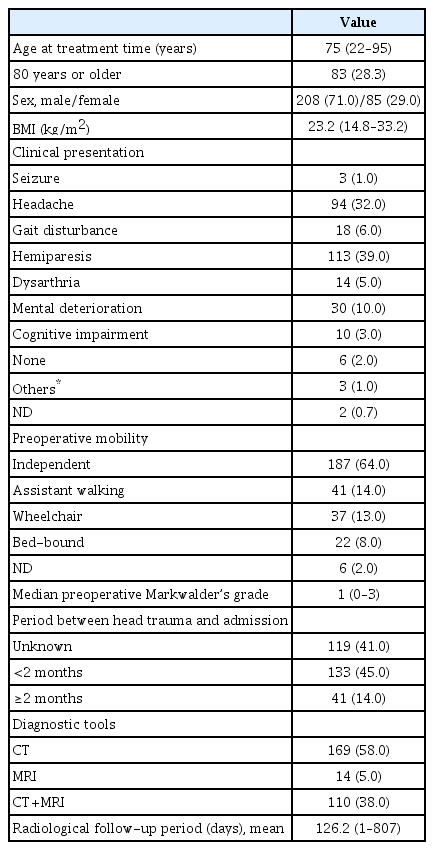
A total of 293 patients were enrolled in this study. There were 208 men (71%), and the median age was 75 years (range, 22–95). Of the 293 patients, 83 (28.3%) were aged 80 years or older. The median body mass index was 23.2 (range, 14.8–33.2), corresponding to the upper limit of the normal range [18]. Hemiparesis and headache were commonly observed symptoms and accounted for 133 (39%) of 293 and 94 (32%) of 293 of complaints, respectively. Seventy-eight percent of the patients were either independent or required assistance while walking before surgery. With a median Markwalder grade [27] of 1, the overall neurological state of the cohort was favorable. Head trauma history was confirmed within 2 months of hospitalization in 133 (45%) of 293 of patients; however, it was not clear in 199 (41%). CT alone or with MRI was used in 279 (95%) of 293 of cases when CSDH was diagnosed. The mean radiological follow-up period was 126.2 days (range, 1–807). The baseline characteristics of the 293 patients are summarized in Table 1.
Recurrence was observed in 17 (5.8%) of the 293 patients. Four of the 17 patients underwent reoperation within 2 weeks of the initial surgery due to an increase in residual hematoma, and mean interval from initial surgery to reoperation was 32.1 days (range, 4–104). On the other hand, in patients whose drainage catheter maintenance period was 1, 2, 3, 4, and 5 days, recurrence occurred in six, five, three, two, and one case, respectively. To identify risk factors for CSDH recurrence, the clinical and radiologic characteristics of the non-recurrence group and the recurrence group were compared and analyzed.
Clinical and radiologic characteristics of patients with CSDH
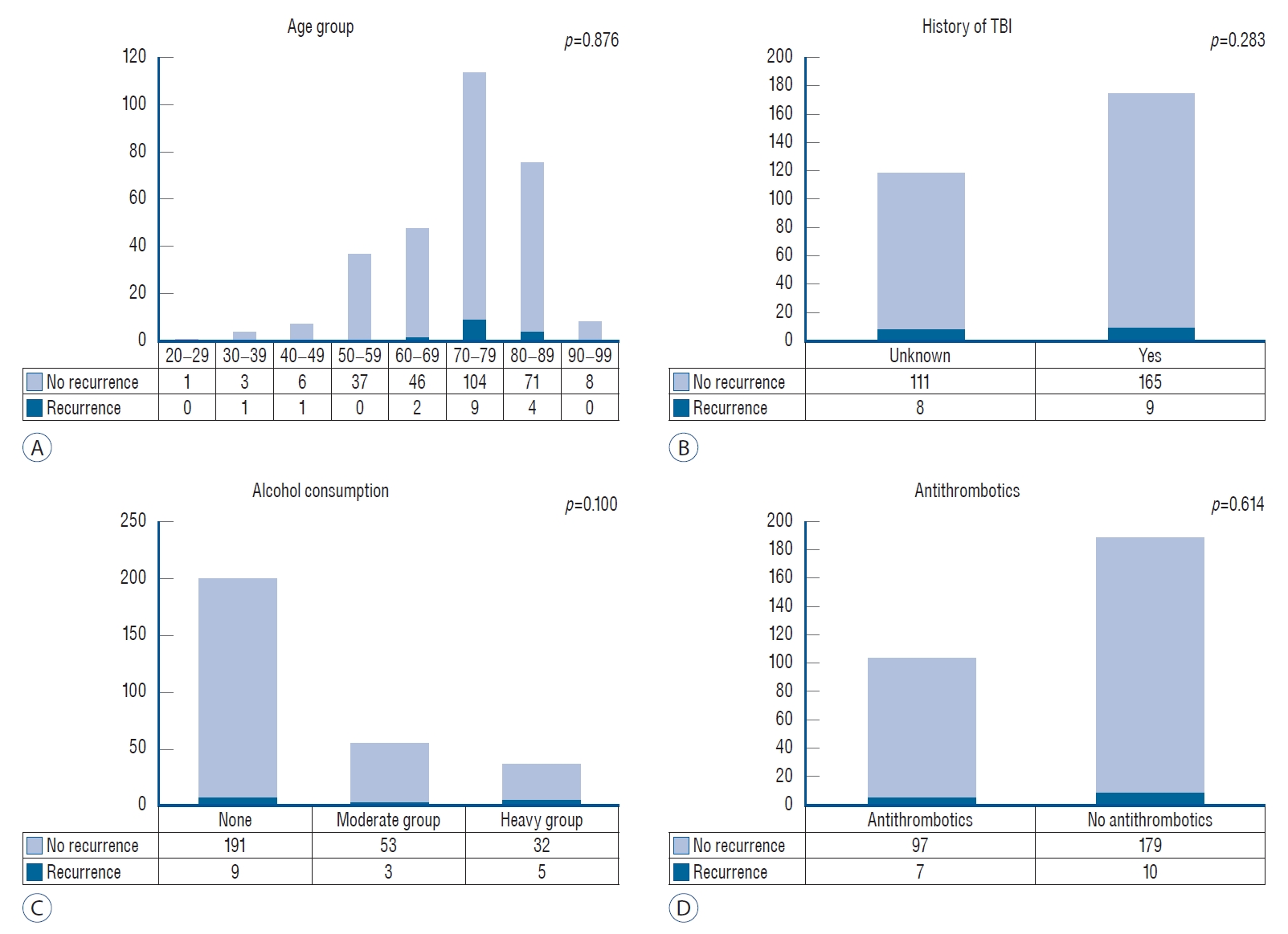
Clinical and radiologic characteristics are summarized and compared in Table 2 and Supplementary Table 1. Two hundred and twenty-seven (77.5%) of the 293 patients had a unilateral hematoma. The most common type of hematoma was the homogenous type (46%). Between the non-recurrence group and the recurrence group, a statistical analysis was performed for each of the following factor : age, sex, preoperative Glasgow coma scale score, head trauma history and period, alcohol consumption, history of smoking, comorbidity, past history of neurological disease, preoperative medication usage, preoperative laboratory findings, and radiological characteristics of CSDH. There were no significant differences between the two groups, except in the degree of alcohol consumption (heavy drinking group, p=0.050). Although there were no statistical differences, several factors showed marginal significance between the two groups : diabetes mellitus (p=0.060), hemoglobin level (p=0.072), activated partial thromboplastin time (p=0.074), and maximum width of hematoma (p=0.075). We also investigated the recurrence rates according to age group, history of traumatic brain injury, alcohol consumption and antithrombotic agents. (Fig. 2). There were no significant differences between each group.
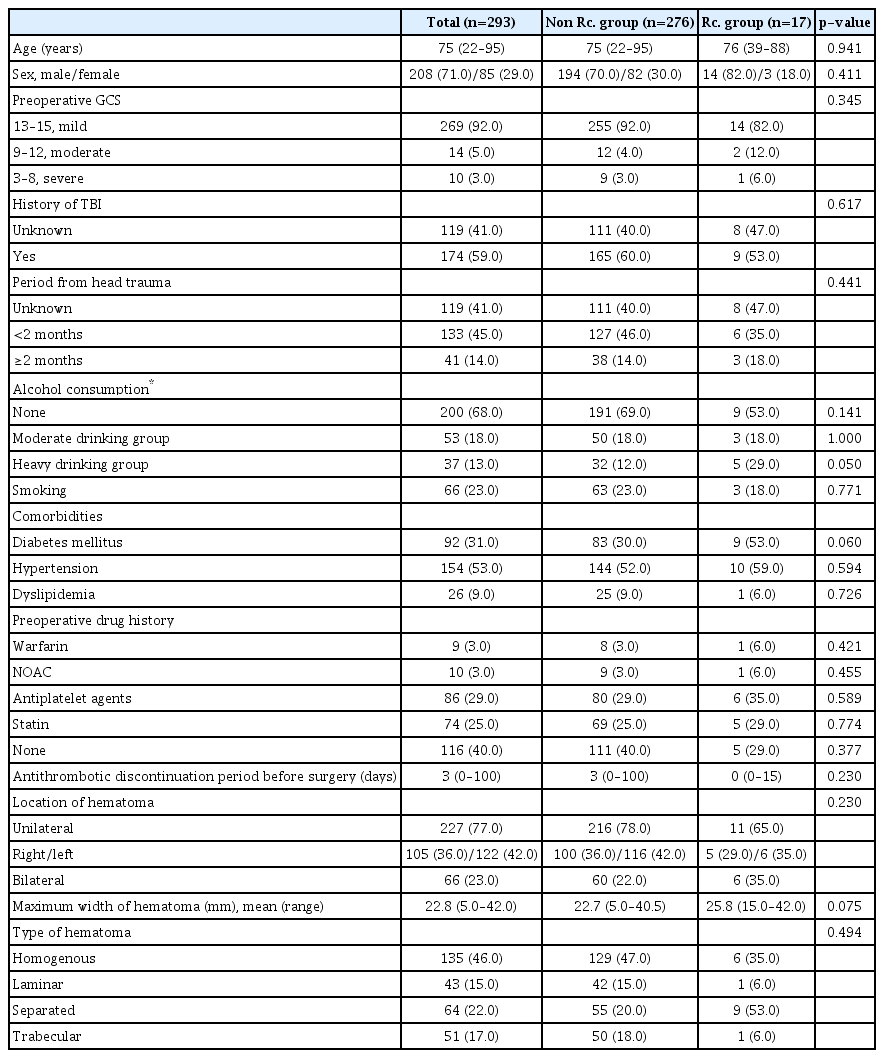
Clinical and radiologic characteristics in non-recurrence group and recurrence group in 293 patients
Surgery-related characteristics of patients with CSDH
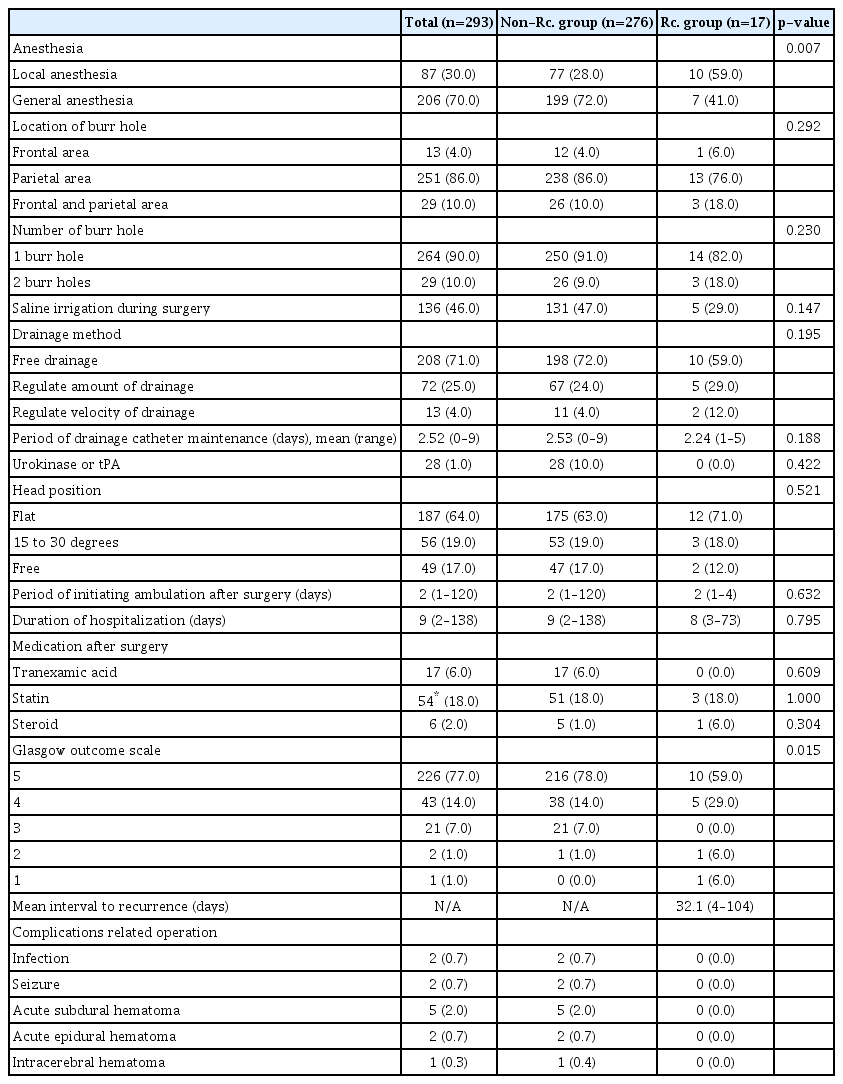
Two hundred and fifty-one (70%) of 293 patients underwent surgery under general anesthesia. Two hundred and sixty-four (90%) of the 293 patients underwent one burr hole trephination. The other parameters are summarized in Table 3. A comparison was performed between the two groups for surgery-related factors and outcomes, including anesthesia type, location of burr hole, number of burr holes, saline irrigation, drainage method, period of drainage maintenance, usage of urokinase or tPA, postoperative head position, ambulation, medication following surgery, symptom improvement, and complications. There were no significant differences between the two groups, except for the anesthesia type and Glasgow outcome scale (Table 3). Surgery was performed under general anesthesia in 72% of patients in the non-recurrence group and 41% in the recurrence group (p=0.007).
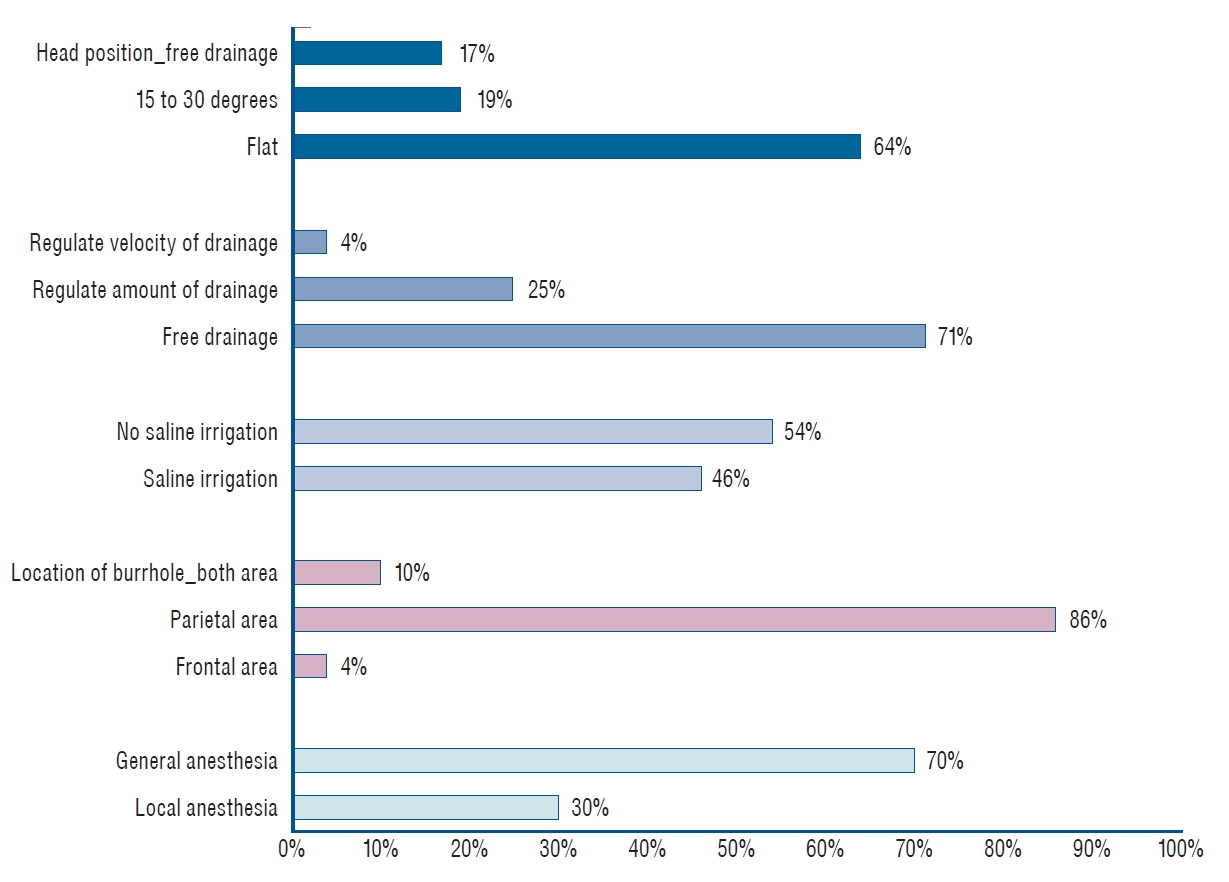
Although there were no significant differences, most of the surgeries were performed with a burr hole in the parietal area, the bed was kept flat, the free drainage method was used, and postoperative symptoms showed favorable outcomes. Postoperative complications, including infection, seizure, acute subdural hematoma, acute epidural hematoma, and/or intracerebral hematoma, occurred in 12 (4.1%) of 293 cases. Surgery-related characteristics in the non-recurrence and recurrence groups are summarized in Table 3. The differences in surgeryrelated methods were also demonstrated in Fig. 3.
Prognostic factors related to CSDH recurrence
Results of the univariate and multivariate analyses of the associations between various possible factors and recurrence were shown in Table 4. Heavy drinking (p=0.042), general anesthesia (p=0.011), and separate type of hematoma (p=0.022) were identified as significant risk factors for recurrence of CSDH in the univariate logistic regression analysis. Other prognostic factors, including antithrombotic agents and laboratory findings, were not statistically significant. A statistical tendency towards a higher rate of CSDH recurrence was observed for age (p=0.066), diabetes mellitus (p=0.056), maximum width of hematoma (p=0.078), hemoglobin (p=0.074), creatinine (p=0.074), and aspartate aminotransferase (p=0.053). In the multivariate logistic regression analysis, general anesthesia (odds ratio, 0.277; 95% confidence interval [CI], 0.097–0.791; p=0.017) and separate type of hematoma (odds ratio, 3.906; 95% CI, 1.275–11.970; p=0.017) were significant risk factors for recurrence of CSDH.
DISCUSSION
CSDH is a common disease found in neurosurgical departments, and its incidence has increased during the last decades [6,36,45]. Particularly, the incidence of CSDH shows an increasing trend in elderly patients [32,37]. Moreover, the global population, including in the Republic of Korea, is growing older [42]. CSDH had a relatively high recurrence rate despite favorable outcomes following surgical treatment. The recurrence rate of CSDH is reported to range from approximately 3% to 30% [15,19,23,36,45]. Therefore, these factors, including incidence, aging population, and recurrence rate, require further attention for CSDH in neurosurgical practice. However, it is difficult to suggest appropriate treatment and follow-up management strategies for CSDH. To date, there is little level I evidence describing optimal perioperative management strategies [23]. Therefore, we, the Clinical Practice Guidelines Committee in Korean Neurotraumatology Society conducted this pilot study to prepare a treatment guideline for CSDH.
In this study, we analyzed the current clinical and radiological characteristics, surgical factors, and outcomes of CSDH in the Republic of Korea through a retrospective multicenter study. The cohort included a majority of elderly male patients. The median age was 75 years, and 71% of the patients were male. Reports show that brain atrophy progresses with age, and the incidence of spontaneous CSDH related to age is significant [24,26]. The high proportion of male patients may be the result of having differences in cranium anatomy, such as size and asymmetry, and activity compared to females [33]. Most of the patients’ preoperative neurological statuses were not severe, with a median Markwalder grade of 1. The common presenting symptoms were hemiparesis (39%) and headache (32%), and the frequency of symptoms was similar to that in the previous reports [8,36,37].
Surgical treatment, especially burr hole craniostomy or trephination, is considered an effective and favorable first-line treatment for symptomatic CSDH. In this study, all patients underwent burr hole trephination and drainage. The effectiveness and safety of burr hole craniostomy for CSDH have been described in many papers [6,14,15,21,43]. Moreover, Ducruet et al. [12] reported that although burr hole craniostomy was associated with relatively higher complication rates, 9.3% in burr hole craniostomy vs. 2.5% in twist drill and 4% in craniotomy, it had significantly lower recurrence rates compared with the other two procedures. On the other hand, in a systemic review and meta-analysis of 34829 patients with CSDH, Almenawer et al. [3] demonstrated that there was no significant difference in mortality, morbidity, cure, and recurrence rates between twist drill and burr hole craniostomy, but craniotomy as a first-line procedure resulted in significantly higher complication rates.
Although this study was conducted with a relatively small number of seven institutions, the preferred surgical method, such as the location of the burr hole, number of burr holes, use of irrigation, and postoperative management including drainage method and patient’s posture was different for each institution or attending physician. In order to develop appropriate clinical guidelines, it is essential to conduct studies on data and consensus from various regions and multi-institutions rather than single-institutional cohort studies, which are susceptible to bias and methodological pitfalls [11]. Therefore, this study was designed as a pilot study to begin the development of Korean guidelines for the management of CSDH.
Some interesting differences in surgical management such as anesthesia method, number of burr holes, and saline irrigation were noted. Among these 293 patients most underwent single burr hole craniostomy. However, 70% of 293 patients underwent surgery under general anesthesia. Sixty-four percent of patients were postoperatively managed with a flat head position, and 71% were managed using the free drainage method. In terms of postoperative head position, Miele et al. [30] and Abouzari et al. [1] reported opposite conclusions about the effect of postoperative head position on the recurrence of CSDH. However, this remains controversial. If this pilot study could be further conducted and analyzed, it would be helpful in establishing treatment guidelines.
As mentioned above, the recurrence rate of CSDH varies. Previous studies have reported that various factors are associated with the recurrence of CSDH. Hematoma density [19,46], postoperative intracranial air [20], and shorter duration of subdural drainage [46] have been reported as independent risk factors. Moreover, there have been many reports that use of antithrombotic agents increases the incidence and recurrence rates of CSDH [10,35,39]. In our study, several prognostic factors that influenced the recurrence of CSDH were identified in the multivariate analysis : general anesthesia and a separated type of hematoma. Surgery was performed under general anesthesia in 70% of the patients. General anesthesia was negatively correlated with CSDH recurrence (odds ratio, 0.277; p=0.0017). Although it is difficult to compare recurrence rates between general anesthesia and local anesthesia groups, previous studies reported that the recurrence rate was 6–15% in CSDH cases treated with local anesthesia [25,28] and 10–20% in CSDH cases treated with general anesthesia [15,22,40]. More recently, Blaauw et al. [7] reported that there is no significant differences in three-month recurrences (12% vs. 10%, p=0.510) between local and general anesthesia. The characteristics of each cohort and the standard anesthesia method of the neurosurgical facilities might be related to the differences in recurrence rate of CSDH. On the other hand, Surve et al. [41] reported that burr hole surgery for CSDH with local anesthesia has significant advantages in terms of operative time, hemodynamic fluctuations, postoperative complications, and length of hospital stay. Therefore, additional research on the difference in the recurrence rate according to the anesthesia method is necessary, and we suggest a reasonable selection of anesthesia according to age, underlying disease, and cooperative ability of patients and others.
In the separated type of CSDH, fibrinolysis occurs in hematoma, and it is known to be related to CSDH recurrence [31,38]. Jack et al. [16] described that the septations in CSDH are associated with larger postoperative residual hematoma collections requiring repeat surgery. More recently, Miah et al. [29] reported a recurrence rate of 14.4% in a systematic review of 22 studies on CSDH. They also reported that there are several radiological prognostic factors of CSDH recurrence, such as hyperdense components, laminar and separated types of hematoma, and bilateral CSDH [29].
Heavy drinking was a significant risk factor for recurrence of CSDH in the univariate analysis but not in the multivariate analysis. It is difficult to find published studies demonstrating a significant association between alcoholism and CSDH recurrence. Kostić et al. [24] reported risk factors for spontaneous CSDH in elderly patients and showed that the percentage of heavy drinkers in the CSDH group was significantly higher than that in the control group. Chen et al. [9] reported that patients with liver cirrhosis and CSDH are significantly more likely to have serious comorbidities, including mortality rate. However, there was no difference in recurrence rate between the liver cirrhosis and control groups [9]. In this study, there was no significant correlation between recurrence of CSDH and liver function laboratory test results. However, due to the small number of patients with liver disease (n=9), statistical significance could not be confirmed. Drain insertion is an important procedure in burr hole trephination. Although we are unable to comment upon the tip position of the drainage catheter, several studies demonstrated that the position of the drainage catheter had no relationship with recurrence rates [34,44]. On the other hand, 71% of the patients with CSDH were male in this study. Oh et al. [33] reported that the size and asymmetry of the cranium were associated with the occurrence of CSDH, and the differences in cranial anatomy may be related to the findings of this study. However, the association between cranium anatomy and recurrence of CSDH is not well understood. To identify the relationship between cranium anatomy and recurrence of CSDH, further studies on this needs to be conducted.
The limitations of this study include those inherent in retrospective analyses and the relatively small number of cases. In our cohort, the quality of medical records varied among the cases at each institute. The retrospective nature of this study might have created a bias. Moreover, this study was conducted as a pilot study to prepare treatment guidelines for CSDH. Seven out of 45 advanced general hospitals in the Republic of Korea were enrolled to analyze the status of clinical management of CSDH for 1 year. Therefore, further study through the participation of more institutions and expansion of the number of patients is mandatory. Moreover, additional studies are also needed to find other detailed risk factors, such as cranial anatomical differences related to CSDH recurrence. In terms of long-term outcomes, several studies have reported that poor emotional or psychological prognosis and cognitive impairment occur approximately 5 years after CSDH treatment [31,38]. Further studies are needed to suggest the long-term management of CSDH. However, despite these limitations, our cohort study is the first multicenter study to analyze patients with CSDH in the Republic of Korea and contributes to the understanding of the current status of CSDH management in the country.
CONCLUSION
This retrospective, the multicenter study reported on the current management and surgical outcomes of patients with CSDH and identified several prognostic factors related to CSDH recurrence. The population characteristics of CSDH are similar to those previously reported. Although there were some differences in the surgical technique and management plan at each institution, the effectiveness of surgical treatment for CSDH was confirmed. Therefore, treatment guidelines based on the various characteristics of cases are necessary. Even though this study has limitations as it was a pilot study, we hope that it will contribute to establishing treatment guidelines for CSDH by providing a scientific basis for further studies.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
INFORMED CONSENT
This type of study does not require informed consent.
AUTHOR CONTRIBUTIONS
Conceptualization : HJO, KC
Data curation : KC
Formal analysis : KC
Funding acquisition : HJO, YS
Methodology : HJO, YS, YIK, KHK, SMK, MHL, KC
Project administration : KC
Visualization : HJO, YS, YHC
Writing - original draft : YHC
Writing - review & editing : HJO, YS, KC
Acknowledgements
This work was supported by funding from the Soonchunhyang University Research Fund and the Yeungnam University Grant (219A580025).
Supplementary materials
The online-only data supplement is available with this article at https://doi.org/10.3340/jkns.2021.0138.
Clinical and laboratory characteristics in non-recurrence group and recurrence group in 293 patients