Surgical Flow Alteration for the Treatment of Intracranial Aneurysms That Are Unclippable, Untrappable, and Uncoilable
Article information
Abstract
Objective
The treatment of complex intracranial aneurysms remains challenging. One approach is the application of surgical flow alteration to treat aneurysms that are neither clippable, trappable, or coilable. The efficacy and limitations of surgical flow alteration have not yet been established.
Methods
Cases of complex aneurysms treated with surgical flow alteration (proximal occlusion with or without bypass, distal occlusion with or without bypass and bypass only) were included in this retrospective study.
Results
Among a total of 16 cases, there were 7 giant aneurysms (≥25 mm diameter) and 9 large aneurysms (>10 mm diameter); 15 of 16 aneurysms were unruptured. There were 8 aneurysms located in the anterior circulation, while the other 8 were in the posterior circulation. Aneurysms were treated with proximal occlusion in 10 cases and distal occlusion in 5 cases; in 1 case, the aneurysm occluded spontaneously after bypass without parent artery occlusion. All but 2 cases underwent prior or concurrent bypass surgery. Complete obliteration of the aneurysm at the latest imaging follow-up was shown in 12 of 16 cases (75.0%). Bypass patency was confirmed in 13 of 15 cases (86.7%). Surgery-related morbidity developed in 3 cases (18.8%, Glasgow outcome scale of 4) and all were perforator infarctions. There were no mortalities.
Conclusion
Surgical flow alteration resulted in a high rate of aneurysmal obliteration with acceptable morbidity. Although several limitations remained, it could represent an alternative method for treating complex aneurysms.
INTRODUCTION
Most cerebral aneurysms can be treated with one of two conventional methods : surgical clipping or endovascular coiling. The trapping of both proximal and distal parent arteries of an aneurysm with or without bypass is another reliable option when a conventional treatment is not feasible. Advances in revascularization using bypass techniques and endovascular intervention tools have made the treatment of more aneurysms possible. However, some complicated aneurysms remain impossible to treat using the methods mentioned above. These troublesome cases include the following features : large or giant aneurysms; dissecting, fusiform, or serpentine aneurysms; recurring aneurysms after coil embolization; perforators or branches that arise from the dome; and other situations that are prone to interruptions in distal flow after treatment10). Flow alteration, also known as "flow diversion" or "flow reduction", has been suggested as an alternative treatment option for these complicated aneurysms152628). The term "flow diversion" has recently been used to refer to the use of flow-diverting stents, and surgical flow alteration can be established by an occlusion at one side of the parent artery, i.e., proximal or distal to the aneurysm. If the mechanism of flow alteration is obscured, the reduction of flow or shear stress into the aneurysm sac with the maintenance of distal flow through collateral arteries or revascularization can be accomplished by bypass surgery. A reduction of intra-aneurysmal flow promotes intraluminal thrombosis and aneurysm occlusion15).
In the last two decades, "open" microsurgery has been used for more complex aneurysms because of advances in endovascular instruments and techniques. Only a few reports have examined such flow alteration techniques and the associated clinical results. We reviewed our experience with flow alteration for complicated aneurysms and discuss the effects, safety, limitations, and possible indications for such cases.
MATERIALS AND METHODS
Patients and surgical flow alteration
The institutional review board of our hospital approved this study. Between January 2008 and December 2013, 3220 cerebral aneurysms (579 ruptured and 2641 unruptured aneurysms) were treated at our hospital. Among these cases, cerebral aneurysms treated using flow alteration techniques were investigated in our current study. A flow alteration technique was defined as incomplete parent artery occlusion that still permitted blood flow into the aneurysm with or without bypass surgery. Aneurysms that underwent trapping, defined as complete proximal and distal flow occlusion, were excluded from this series. Aneurysms that developed at the extradural or cavernous internal carotid artery (ICA) were also excluded. We identified 16 cases of aneurysms (0.49%) that were treated by surgical flow alteration and retrospectively reviewed patient medical records and images.
The surgical methods for obliterating the aneurysm and for creating the bypass for revascularization in each case were investigated and analyzed. Occlusion methods were classified into three subgroups : proximal occlusion only, distal occlusion only, and no occlusion. In cases of partial (one of two) distal occlusions in addition to proximal occlusion [for example, occlusion at ICA and proximal anterior cerebral artery (ACA), but not at middle cerebral artery (MCA) for an ICA bifurcation aneurysm], patients were also classified into the "proximal occlusion only" subgroup because distal flow via the bypass still remained in those cases. The methods and results of each bypass were assessed. Representative cases of various flow alteration methods were each described in a separate session. Obliteration of the aneurysm and patency of the bypass were evaluated. Any surgery-related complications were also reported.
Preparation, treatment and follow-up
Angiography was performed prior to surgery in all cases. When bypass surgery was required, the external carotid artery was also included in the examination. Angiography for the radial artery in the non-dominant arm was evaluated in cases when bypass using a radial arterial interposition graft was expected. During angiography, the collateral circulation capacity was evaluated as necessary : balloon test occlusion (BTO) of the contralateral ICA was used for anticipated ICA occlusion. Otherwise, occlusion of the contralateral vertebral artery (VA) was used in preparation for VA occlusion. When balloon test occlusion was deemed tolerable, single-photon emission computed tomography (99mTc-HMPAO SPECT) was performed to verify the safety of occlusion. Intraoperative monitoring of the evoked potential was performed in all but one case of mechanical monitoring failure. The patency of the vessels, especially of the bypass grafts, was evaluated using microvascular Doppler flowmetry and indocyanine green videoangiography.
Computed tomography (CT) angiography and perfusion CT were performed immediately after surgery to evaluate acute-stage patency of the bypass, the state of the aneurysm, and immediate complications. Surgical outcomes were expressed using the Glasgow outcome scale (GOS) at the time of discharge and at follow-up. The follow-up schedule was planned individually based on clinical and aneurysmal status. The final imaging follow-up was evaluated using angiography, CT angiography, or magnetic resonance (MR) angiography.
RESULTS
Characteristics of patients and aneurysms
There were 13 women and 3 men with a mean age of 51.1 years (range, 26-65 years). Aneurysms were unruptured before treatment in 15 of 16 cases. The most common symptoms were non-focal, including mild headache and dizziness (8 patients, 50%). The second most common symptom was cranial nerve problems, including diplopia and blindness (2 patients, 12.5%). Additionally, two cases of regrowth of previously coil-embolized aneurysms were included. The other characteristics of each patient and aneurysm are summarized in Table 1.
Aneurysms were located in the anterior circulation arteries in eight cases, including three cases involving the ICA and four cases involving the MCA, including the anterior temporal artery (ATA) and one case in the distal ACA. The other eight aneurysms involving the posterior circulation arteries were located at the basilar artery (BA), posterior cerebral artery (PCA), superior cerebellar artery (SCA), and junction of the vertebral artery (VA) and posterior inferior cerebellar artery (PICA). Giant aneurysms (≥25 mm, maximum diameter) and large aneurysms (≥10 mm, maximum diameter) developed in seven (43.8%) and nine (56.3%) patients, respectively. There were nine saccular aneurysms, three fusiform (including serpentine type) aneurysms, and four dissecting aneurysms.
Surgical flow alteration (Table 2, 3)
Proximal occlusion was performed in 10 cases, including all ICA aneurysms and basilar tip aneurysms. Proximal occlusions were performed with surgical clipping in nine cases, and with endovascular embolization in one case (case 2). Distal occlusion was performed in five cases. One case of basilar trunk aneurysm was occluded by endovascular embolization, while the others were occluded surgically. Occlusion was not performed in one case of a VA dissecting aneurysm involving the PICA because the aneurysm spontaneously occluded following the occipital artery (OA)-PICA bypass.
Bypass surgery was performed in 14 patients. In case 2, two rounds of bypass surgery were required because of acute occlusion of the primary external carotid artery (ECA)-saphenous venous interposition graft (SVG)-insular segment of the MCA (M2) high-flow bypass, which was followed by revision with a superficial temporal artery (STA)-radial artery interposition graft (RAG)-M2 bypass. The STA (stump or its distal branches) was most commonly used as a donor. RAG was used in six cases : as a long graft for a high-flow bypass (case 1) and as a short graft between the STA-RAG-MCA (cases 2, 3, and 7) and STA-RAG-SCA (cases 9 and 14). Double-barrel bypasses were performed in the STA and the distal branches of the ACA (case 4) and the STA and the two cortical segment branches of MCA (M4) (case 8). Bypass surgery was not performed in two cases of basilar top aneurysms with both posterior communicating arteries (PCoAs) greater than 1 mm in diameter.
Case presentation
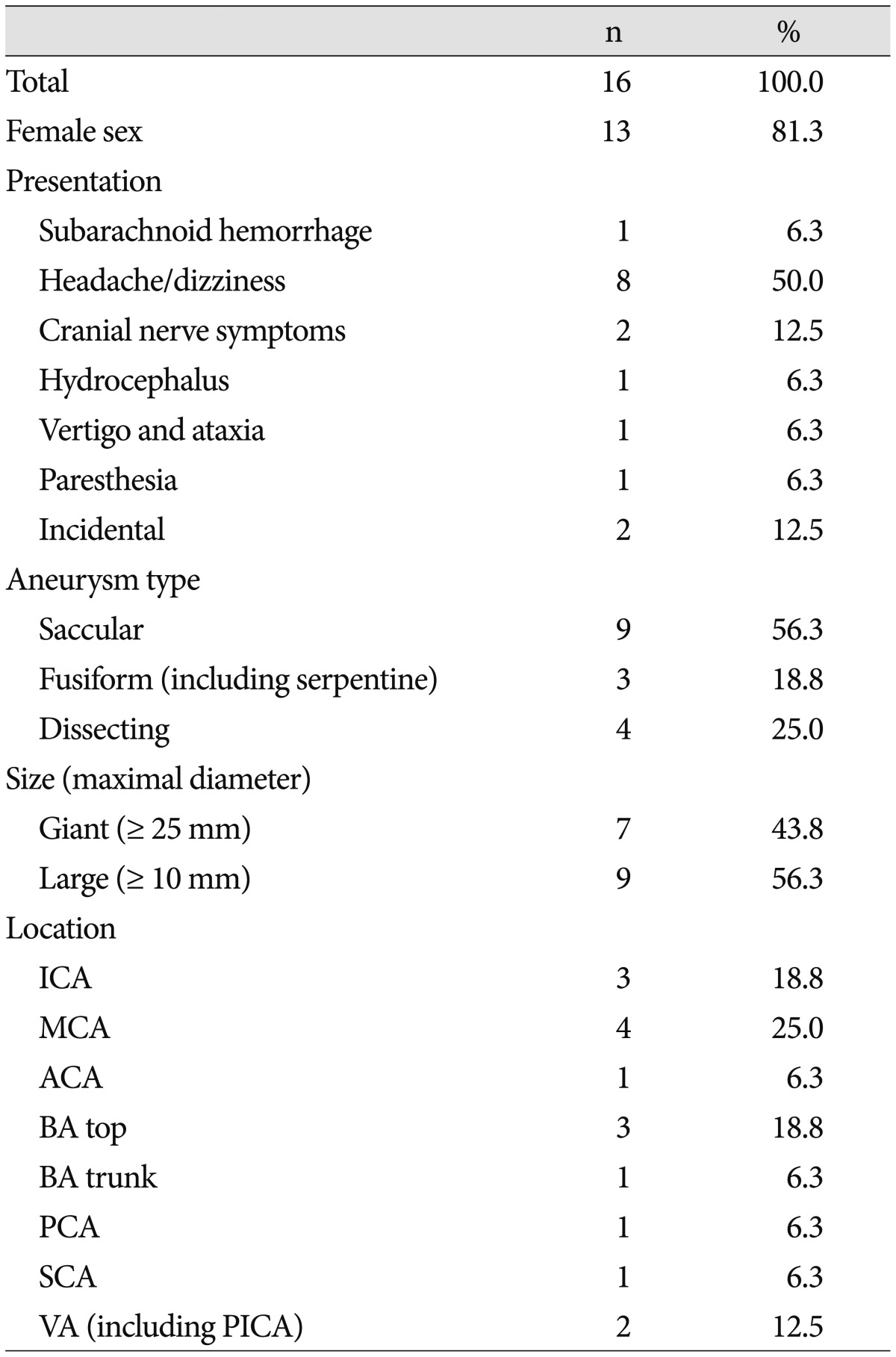
Case 4 : proximal occlusion with bypass (Fig. 1)
Proximal occlusion with bypass (Case No. 4). A : An MR T1-weighted sagittal image suggestive of a giant fusiform aneurysm of the left anterior cerebral artery (ACA) with a thrombus compressing the genu of the corpus callosum. B : Lateral view of left internal carotid angiography showing a giant serpentine aneurysm that developed from a post-communicating segment of the ACA (A2) along its distal segments. C : 3D-reconstructed angiography revealing the frontopolar artery arising from the aneurysmal sac (arrow). Right A2 (arrowhead) is deviated to the opposite side because of the mass effect. D : Indocyanine green videoangiography that was performed to follow dual anastomosis showing patency from the frontal (arrow) and parietal (arrowhead) branches of the superficial temporal artery (STA) to the cortical branches of the distal ACA. E : 3D-angiography at postoperative day (POD) 4 showing proximal occlusion was performed by clipping the left A2 immediately distal to the anterior communicating artery and right A2 (arrowhead). F : The frontopolar artery (arrow) is filled in a retrograde fashion through the bypass in a left external cerebral artery angiography at POD 4. The aneurysmal sac is minimally stained via the frontopolar artery. Anastomosis of the parietal branch of the STA-distal ACA is not observed.
A 26-year-old woman presented with gradually aggravated headache. MRI and MR angiogram showed a giant thrombosed aneurysm at the post-communicating segment of the ACA (A2) portion with mass effect. Angiography revealed a giant fusiform aneurysm of the left ACA, which was 6.5×1.8 cm in diameter, and developed from the proximal A2 and continued to the pericallosal artery. The frontopolar artery arose from the proximal aneurysm sac. The patient underwent left STA-distal ACA bypass via a bicoronal midline approach, and two small separate craniotomies were performed for Lt STA-distal ACA bypass. Both the frontal and parietal branches of the STA were successfully connected to two different distal ACA branches using 11-0 nylon in an end-to-side fashion. A proximal A2 immediately distal to the anterior communicating artery (ACoA) was then occluded by a clip via another craniotomy for the pterional approach.
Her postoperative clinical course was excellent without any neurologic deficits. Angiography on postoperative day (POD) 4 showed a minimal amount of retrograde filling of the aneurysm through the frontopolar artery via the frontal branch of the STA. Flow through the parietal branch of the STA was no longer visualized. A CT angiogram after 12 months did not show any remnant sac or intact patency of the bypass through the frontal branch of the STA.
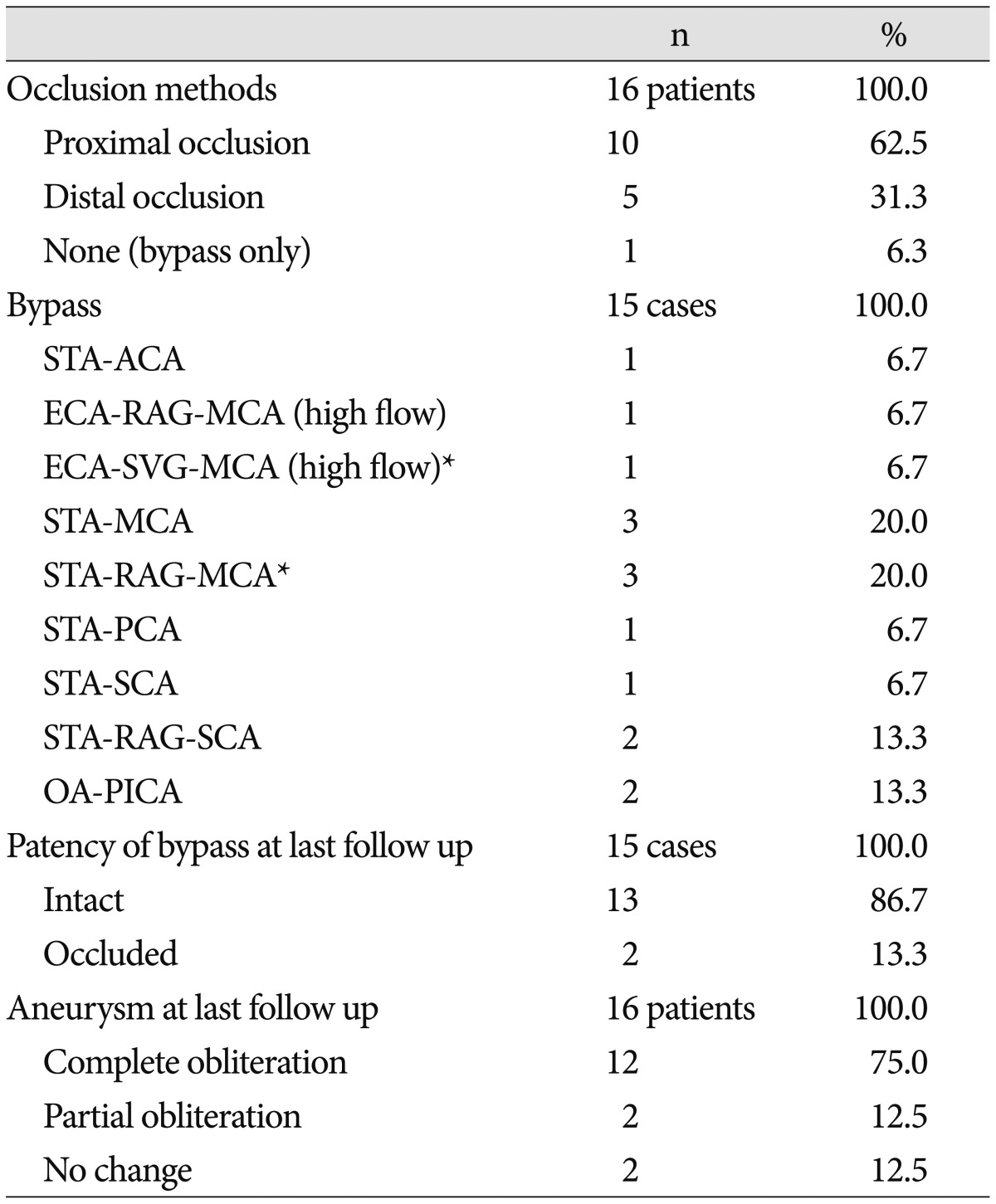
Case 12 : proximal occlusion with bypass (Fig. 2)

Proximal occlusion with bypass (Case No. 12). A : 3D angiography showing a basilar artery (BA) aneurysm with a broad neck and involvement of the left posterior cerebral artery (PCA) and superior cerebellar artery (SCA). B : Angiography at the end of coil embolization suggesting the successful obliteration of the aneurysm. C : An MR angiogram obtained two months later revealing compaction of the coil and regrowth of the sac. D : An intraoperative photo showing end-to-side anastomosis of the parietal branch of the left superficial temporal artery (STA, arrow) and ipsilateral SCA (arrowhead), followed by proximal occlusion by clipping of the distal BA. E : An anterior-posterior view of the vertebral angiography at postoperative day 3 showing occlusion at the distal BA. F : External carotid angiography at postoperative day 3 showing near-total obliteration of the aneurysm and intact patency of both SCAs and both PCAs through the bypass (arrowhead) and the posterior communicating artery.
Multiple aneurysms were found incidentally in a 65-year-old female. The lesions arose from the left pre-communicating segment of the ACA (A1), bifurcation of the left MCA, and the top of the BA. Although the former two aneurysms were clipped via a left pterional approach, direct clipping the BA aneurysm was not feasible because of its high location, broad neck, and the involvement of the left PCA and SCA. Therefore, coil embolization for BA aneurysm was performed in this patient. An MR angiogram obtained on the first day of embolization suggested the successful obliteration of the aneurysm; however, a follow-up MR angiogram obtained two months later showed coil compaction. The patient underwent flow alteration by proximal occlusion and revascularization of the distal flow. Because neither of her PCoAs were large enough, bypass surgery was required. In the setting of a lateral decubitus position for a subtemporal approach, the parietal branch of the left STA was harvested and connected ipsilateral to the SCA in an end-to-side fashion using Nylon 10-0. Proximal occlusion was performed by clipping the distal BA.
Her postoperative course was uneventful. Angiography at POD 3 showed minimal change in the remnant sac and intact patency of the bypass through the SCA to both the PCA and contralateral SCA. An MR angiogram taken four months after surgery suggested that the remnant sac of the aneurysm was no longer visible and that the patency of the bypass was intact.
Case 7 : distal occlusion with bypass (Fig. 3)
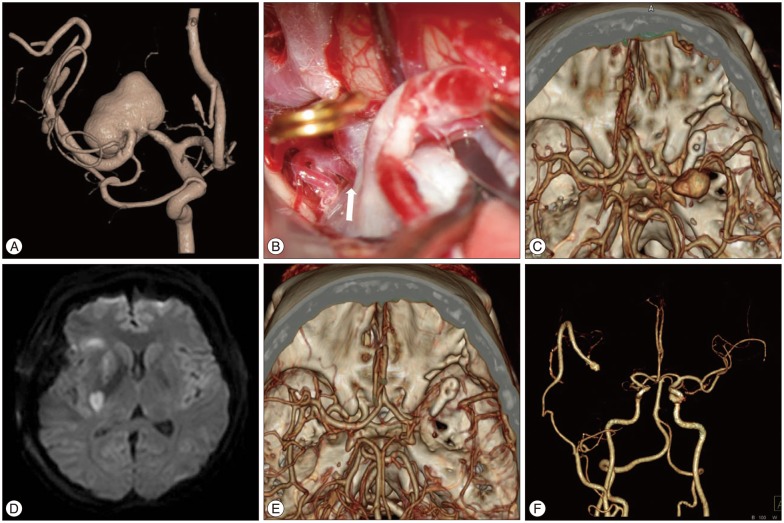
Distal occlusion with bypass (Case No. 7). A : 3D-reconstructed angiography showing an aneurysm of the middle cerebral artery (MCA) 20×11 mm in size with a broad neck. B : In the operative view, a large medial lenticulostriate artery (arrow) developed from the medial side of the aneurysm. C : Brain CT angiography taken immediately postoperatively showing distal occlusion of the parent artery and a smaller, but still visible, aneurysmal sac. The distal MCA is filled in retrograde fashion via a superficial temporal artery-radial artery interposition graft-insular segment of the MCA bypass. D : MR diffusion performed on the second day after surgery suggesting acute lacunar infarction at the right basal ganglia and corona radiata. E : In a CT angiogram obtained on postoperative day 7, the aneurysmal sac is not visualized. F : In a CT angiogram taken 20 months after surgery, patent bypass flow and no recurrence of the aneurysm were observed.
A 37-year-old female patient presented with temporary paresthesia on her right side. During evaluation for possible transient ischemic attack, an MRI showed a large aneurysm in the right horizontal segment of the MCA (M1). Conventional angiography showed that the aneurysm was 20×11 mm in size with a broad neck and an elongated and lobulated configuration. Because the aneurysm was large in size, involved the M1, and perforated the originating sites, direct clipping or endovascular embolization was not feasible. We planned to trap and revascularize the distal flow via bypass. Her radial artery was harvested for an interposition graft, as previously described, because her STA branches were sufficiently large.38) The patient underwent surgical exploration via a pterional approach. The RAG was connected to the stump of the STA in an end-to-end fashion and the other end of the RAG was connected to the frontal M2 in an end-to-side fashion using 10-0 nylon. However, we found that a large medial lenticulostriate artery originated from the proximal portion of the aneurysm sac and made it impossible to perform a proximal occlusion. Only the distal M1 was occluded by clipping.
Mild hemiparesis of the left side was detected upon immediate postoperative neurological examination. However, CT angiography showed good visible patency of the MCA via the bypass. Two days later, her hemiparesis was suddenly aggravated. MR diffusion revealed an acute lacunar infarction at the right basal ganglia and corona radiata. Her hemiparesis was still present, but improved with time (GOS 3 at discharge). Follow-up angiography 30 days after surgery showed that only the proximal part of the aneurysm could be visualized and that the bypass flow was patent. The remnant sac was not visible on CT angiography at the five-month or two-year follow-up after the surgery. Her hemiparesis was also greatly improved to a near-normal state (GOS 4 at 2 years) at the last follow-up.
Surgical outcomes
The mean imaging follow-up was 20.4 months (range, 6-52 months). Among a total of 16 patients, greater than 6 months of follow-up was possible for all but one case (due to patient preference), and was greater than 1 year for 12 cases (75%) and greater than 2 years for six cases (37.5%). Complete obliteration of the aneurysm at the final imaging follow-up was observed in 12 of 16 cases (75.0%). In case 5, the MCA serpentine aneurysm was almost completely obliterated after distal occlusion and bypass, and only a small residual proximal stump remained at 24 months, as previously reported41). However, regrowth was observed 36 months after occlusion and the patient underwent additional coil embolization. There were two cases with no changes of the aneurysmal sac until 3 or 24 months after proximal occlusion. Both cases involved a BA top aneurysm, which similarly involved both PCAs and SCAs in the sac. Bypass was not performed concurrently in these cases because both of the PCoAs were large.
The patency of the bypass remained intact in 13 of 15 cases (86.7%). There was a 'silent' occlusion of a SCA fusiform aneurysm in case 14. The patient underwent proximal SCA occlusion with STA-RAG-SCA bypass. The bypass flow disappeared with time, and CT angiography obtained 12 months postoperatively showed neither flow nor infarction. No neurological deficit developed in that case.
Morbidity
The mean GOS at discharge was 4.56 (range, 3-5), while the median GOS was 5, including the preoperative neurological deficits that had developed. Among 16 patients, surgery-related morbidity occurred in 3 cases (18.8%), including in case 7 as described above. In case 2, acute occlusion of the bypass occurred at the SVG and immediate revision with a STA-RAG-M2 bypass was needed. There were no other neurological deficits immediately after two rounds of bypass surgery; however, motor aphasia occurred six days later after endovascular occlusion of the proximal ICA (ophthalmic segment). MR revealed an acute infarction of the left periventricular white matter. Other morbidities developed in case 10 of BA top aneurysm that involved both PCAs and both SCAs within its broad neck. The patient underwent proximal occlusion at the distal BA without bypass because of the large PCoAs at both sides. Evoked potential monitoring was attempted, but failed because of a mechanical error. Proximal occlusion was performed by direct clipping after confirming that there was no visible perforator. Despite these efforts, postoperative hemiparesis developed immediately due to pontine infarction. There was no surgical mortality in this series.
DISCUSSION
'Unclippable' complicated aneurysms
Although the term 'unclippable aneurysm' is difficult to define, it includes aneurysms that are very large or giant in size, fusiform or serpentine in shape, and those that give rise to branches. Several MCA or BA aneurysms can also be unclippable, especially when many branches or perforators are related. These unclippable aneurysms have a poor clinical prognosis because of the high recanalization risk despite several potential methods of treatment and treatment-related morbidity19). The natural course of these aneurysms is also known to be unfavorable. Giant aneurysms have been reported to have a high risk of rupture, thrombosis of perforators, and mass effect1135). Although there have been some reports of the spontaneous regression of large serpentine aneurysms1732), cases have also shown the opposite result, including abrupt neurological deterioration and death25) or recanalization that became thrombosed22). Consequently, several studies have strongly recommended the most aggressive treatment possible for these complicated aneurysms2336).
The treatment of a complicated aneurysm should be individualized. However, the common goal of the treatment for a cerebral aneurysm is obliteration of the lesion with maintenance of distal cerebral blood flow. When the standard simple treatment option, clipping or endovascular embolization, is not feasible, several alternative methods have been introduced. Notably, revascularization using a bypass technique has made additional treatment options possible30). Trapping with bypass could be a treatment of choice for selected unclippable aneurysms because certain methods for obliterating a lesion can minimize the risk of ischemic stroke1020). Trapping of the parent artery can be accomplished with both surgical and endovascular techniques1). Resection of the aneurysm and end-to-end anastomosis of the parent artery or other various in situ bypass methods have also been introduced36243031). Reconstruction of an aneurysm using multiple clips is another option for very select cases, but is considered to be technically challenging and with limited indications21). Wrapping with muslin gauze or cotton is still widely used278). Although some authors have introduced wrapping and reinforcing with the application of a clip, which is thought to improve its preventive effects, wrapping is generally not recommended as a primary treatment modality because of its uncertain preventive effects against rupture412).
Surgical flow alteration
When several methods of obliteration are not feasible for the treatment of a complicated aneurysm, flow alteration can represent an alternative option. In many cases in this series, surgical flow alteration was the 'Plan B' secondary to direct clipping or trapping during surgical planning. Flow alterations were performed when direct clipping, endovascular embolization, or trapping was not possible. There were various reasons for performing flow alteration in this series. The most common situation was when distal branches or crucial perforators originated directly or immediately adjacent to an aneurysm. For example, distal occlusion alone was performed in case 5 because the ATA arose from an area immediately proximal to the aneurysm. The aneurysms included PCAs, BA, and/or SCAs in cases 10, 11, and 12. Another situation was when the mass of the large or giant aneurysms (cases 1, 2, 7, and 14) or another adjacent aneurysm (case 8) interfered with the identification of the neck of the aneurysm or the proximal or distal artery site. Additionally, adhesions of the aneurysmal sac and adjacent structures could make trapping impossible (case 3).
The goal of flow alteration is to achieve spontaneous thrombosis of an aneurysm by reducing wall shear stress in the aneurysmal sac. Flow alteration could be made by a one-sided (proximal or distal) occlusion of the parent artery with or without bypass. Among several different methods for flow alteration, proximal occlusion with bypass is relatively well-established. Kalani et al.18) reported that occlusion of the proximal flow can diminish intraluminal pressure, which reduces the risk of rupture, whereas slow retrograde flow induces thrombosis within the aneurysmal sac. We also preferred proximal occlusion, which we performed in more than half of the cases in this study. In this case series, the complete obliteration of the aneurysm resulted from a proximal occlusion in eight of ten cases. Among two non-obliterated cases, both were BA bifurcation aneurysms that had undergone occlusion of the distal BA without bypass because of the large size of both PCoAs. We thought that continuous flow from the PCoAs to the PCAs, SCAs, and perforators might interrupt thrombus formation in the sacs. There have been a few reports suggesting that an aneurysm at a basilar bifurcation can become enlarged, despite proximal occlusion by clipping or embolization at a distal basilar artery, such that it therefore requires additional surgical interventions2837). Both patients were followed with observation only because the aneurysm size remained stable on neuroimaging follow-up. However, the possibility of enlargement remains, so careful long-term follow-up is required.
Hunterian ligation, a classical flow diverting option for aneurysms, is an older method than the clipping technique11). This technique, a type of proximal occlusion, has been applied to treat giant or other complicated aneurysms of the ICA by cervical ICA ligation. Hunterian ligation diminishes flow into aneurysms of various locations and promotes thrombosis formation. However, Hunterian ligations without revascularization resulted in a high rate of complications, because approximately one-fifth of the population cannot tolerate abrupt occlusion of an ICA1319). Drake et al.11) reported satisfactory treatment of giant aneurysms using Hunterian ligation with bypass. They suggested that preoperative evaluations to determine whether bypass was required were crucial for obtaining good outcomes. In this present case series, all three cases of distal ICA large or giant aneurysms were successfully treated by occlusion of the cervical ICA and bypass. Among these, two patients who did not pass BTO underwent high-flow bypass, while the other patient (case 3) had passed BTO and SPECT and underwent low-flow (STA-MCA) bypass.
Surgical flow alteration with proximal occlusion could not be applied in some cases because the aneurysm size was too large, firm adhesions of the sac interfered with exposure of the proximal artery, critical perforators had arisen, or there was no space for clipping. Occlusion of the distal flow with bypass could represent an additional option in such situations. Horowitz et al.16) reported a case of a giant serpentine aneurysm of the MCA that was successfully treated using a single clip immediately distal to the dilated artery with STA-MCA bypass. Nussbaum et al.29) reported three cases of dissecting aneurysm at the PICA to which wrapping or bypass could not be applied, so they were managed by remote distal outflow occlusion without bypass. The authors suggested that occlusion of the distal flow results in immediate elevation of the aneurysm intraluminar pressure, followed by a subsequent reduction in pressure. In this case series, there were five cases of distal occlusion, including three cases of MCA aneurysm, and one case each of BA trunk and SCA dissecting aneurysms. These did not rupture during distal occlusion or the follow-up period; however, one patient (case 5) had a proximal remnant sac that emerged during follow-up and underwent additional endovascular coil embolization at 36 months after surgery.
In this case series, case 15 underwent OA-PICA bypass prior to embolization of the dissecting aneurysm of the VA that had fortunately thrombosed. There are several reports of spontaneous aneurysmal thrombosis following bypass of the distal flow5141940). Authors of previous studies have suggested that partial thrombosis was facilitated by revascularization of the bypass. However, there is a risk in suggesting bypass alone as a standard treatment option. Because the mechanism is not yet established, the results remain uncertain, and the lesion is typically life-threatening.
Similarly, other techniques of flow alteration also have limitations. Isla et al.18) reported the regrowth of an MCA serpentine aneurysm two years after its obliteration following a STA-MCA bypass and ICA ligation. In a series of MCA giant aneurysms by Kalani et al.19), 18.8% of patients who underwent proximal or distal occlusion with bypass showed a remaining sac that required additional treatment. Those authors suggested that life-long follow-up is required for patients who undergo flow alteration. Cases of fatal rebleeding after occlusion of the basilar artery for the treatment of a giant aneurysm of the basilar apex have been reported3439). The authors proposed a simulated hemodynamic model based on evidence that the proximal occlusion can result in jet flow through the PCoA into the sac, which promotes fatal aneurysmal rupture. Moreover, there have been reports that bypass surgery for distal flow might promote the regrowth of an aneurysm19) or even perioperative aneurysmal rupture33).
Bypass surgery
Indications for bypass in surgical flow alteration remain controversial. BTO is a useful method for helping physicians to understand whether a patient can tolerate occlusion without bypass. However, we did not routinely perform BTO because of its own risk of complications; moreover, passing BTO does not always indicate that a patient is infarction-free27). Our policy is to perform bypasses when possible, even when a small risk of ischemia is expected following occlusion. When the ischemic risk following occlusion is obscure, bypass surgery is decided by comparing its risk and expected effects. The type of bypass is determined by the demand of the vascular territory prone to be occluded and supplied from the collaterals. When an occlusion was limited to part of an MCA territory, a STA-MCA [opercular segment (M3) or M4] bypass was favored. When the entire MCA territory was occluded, STA-short interposition grafts, usually using a radial artery-MCA (proximal to M3) bypass, was primarily considered. However, the caliber of the STA was also a critical factor in selection. High-flow bypass was considered when the graft was needed to supply beyond the MCA territory or when collateral thorough the ACoA or PCoA was poor. When PCAs were of a fetal origin, a bypass that carried more flow than that described above was required. However, the final selection of the bypass type was critically influenced by the state of the recipient arteries. When a recipient artery was not large enough for the expected demand, a double-barrel bypass was the next choice.
Morbidity and limitations
Surgery-related morbidity in this series occurred in 3 of 16 cases (18.8%); all were infarction of the perforator territories, including two cases of lenticulostriate arteries and one case of pontine perforators. The delayed ischemia observed in case 2 suggested the risk of embolization to the perforators induced by intramural thrombosis. In all cases of surgical morbidity, the aneurysm size was very large or giant. Although the complication rate was improved compared to previous reports, treatment of a giant aneurysm remains challenging and risky 91020). In a previous report of flow alterations by Hoh et al.15), procedure-related morbidity and mortality were 6.3% and 10.4%, respectively. The obliteration and morbidity rate of the present series was acceptable in comparison with other studies. However, there are several limitations to this study, including the limited number of cases, its retrospective study design, the diverse types and various locations of the aneurysms, and cases with a short follow-up period.
CONCLUSION
Flow alteration can be performed as an alternative treatment method for unclippable complex aneurysms. Complete obliteration was obtained in 75.0% of our study cases after surgical flow alteration. Moderate surgery-related morbidity developed in 3 of 16 patients and there were no deaths in our current case series. Surgery-related complications included infarction of the perforator territories that developed in giant or very large ICA aneurysms, and broad and obscured necks of basilar top aneurysms. The treatment of complicated aneurysms should be individualized because the hemodynamics, state of the collateral circulation, and surgical locations are each unique. Understanding the nature of the cerebral circulation is also crucial for obtaining a successful result for recent flow alteration. For the successful treatment of an atypical or very large-sized aneurysm, meticulous preoperative preparation for various types of planning is crucial because "real" situations can only be identified after surgical exploration.