Delayed Appearance of Radiologically Occult Cerebral Arteriovenous Malformation : A Case Report and Literature Review
Article information
Abstract
It is critical to identify the ruptured cerebral arteriovenous malformations (AVMs) for secondary prevention. However, there are rare cases unidentified on the radiological evaluation. We report on a patient with the delayed appearance of radiologically occult AVM as a probable cause of the previous intracerebral hemorrhage (ICH). An 18-year-old male patient presented with a right temporal ICH. The preoperative radiological examination did not reveal any causative lesions. Because of the intraoperative findings suggesting an AVM, however, only hematoma was evacuated. Disappointedly, there were no abnormal findings on postoperative and follow-up radiographic examinations. Eleven years later, the patient presented with an epileptic seizure, and an AVM was identified in the right temporal lobe where ICH had occurred before. The patient underwent partial glue embolization followed by total surgical resection of the AVM and anterior temporal lobe. Based on the literature review published in the era of magnetic resonance imaging, common clinical presentation of radiologically occult AVMs included headache and seizure. Most of them were confirmed by pathologic examination after surgery. In cases of the ICH of unknown etiology in young patients, long-term follow-up should be considered.
INTRODUCTION
Cerebral arteriovenous malformations (AVMs), characterized by the feeding arteries, nidus and draining veins, are a congenital disease [19,28]. Their most common and serious presentation is the intracerebral hemorrhage (ICH) at the age between 20 and 40 years old [9,28]. So, it is general to examine the probably accompanying AVM when ICH occurs in young patients [9,33]. AVMs are easily diagnosed with radiological tools such as computed tomography (CT), magnetic resonance (MR) imaging and digital subtraction angiography (DSA). However, there is a rare entity of occult AVMs, also called crypt AVMs, which are histologically present but invisible on radiologic examinations [4,22]. With the development of brain MR imaging, the identification of occult AVM has significantly improved [13]. Nonetheless, there are still occult AVMs not detected with DSA and brain MR imaging. We are to introduce a case presenting with cryptogenic ICH 11 years ago and epilepsy recently which were caused by radiologically occult AVM, with review of the literature.
CASE REPORT
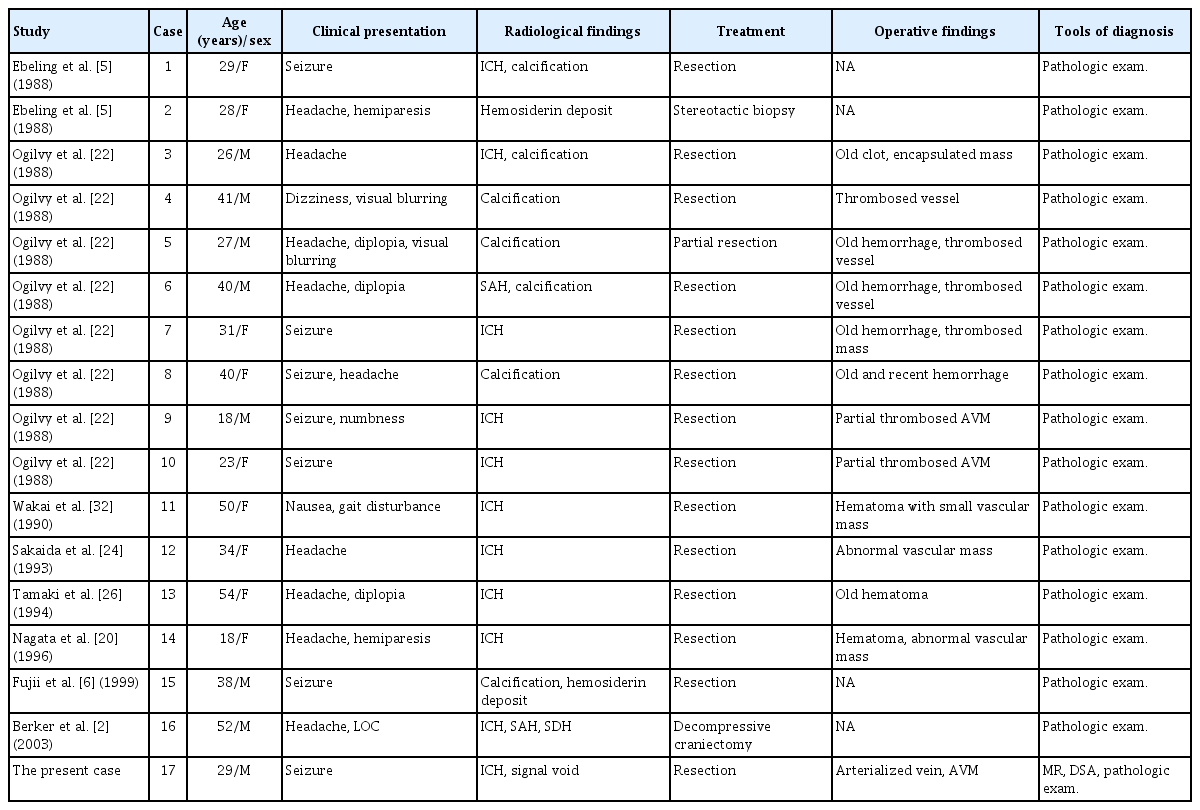
An 18-year-old male patient with no underlying medical history presented with a sudden severe headache, vomiting and altered mentality. On the radiological examination, a 45-cm3 sized ICH was identified in the right temporal lobe with a 9-mm midline shift to the left. The patient underwent craniotomy and ICH removal. During the surgery, an arterialized vein was identified on the temporal surface (Fig. 1), suspected of a shunt of vascular malformation. Hematoma evacuation alone was done, remaining the abnormal vascular lesion, in order to escape from the catastrophic situation such as AVM rupture without grasping the angioarchitecture. On the postoperative DSA, however, there was no clear evidence of vascular malformation except a slow shunt flow which was identified later. There was no evidence of AVM on the pathologic examination. Antiepileptic drug was discontinued in 7 months after surgery, however, it was restarted in 5 months due to the occurrence of epileptic seizure. Brain MR imaging at that time showed no abnormal findings except the cerebromalacia in the right anterior temporal lobe (Fig. 2A). Antiepileptic drug was discontinued again in 10 months. Thereafter, the patient was lost to follow up on his own will, during which the patient said that there was no seizure event.
A and B : The initial brain computed tomography and magnetic resonance imaging showed intracerebral hemorrhage in the right temporal lobe with no other causative lesions. C and D : There was no abnormal cerebrovascular lesions on the preoperative digital subtraction angiography (DSA). E : During the surgery, arterialized vein (arrows) were identified. F and G : On the postoperative DSA, there was no clear evidence of abnormal vascular lesion but a suspicious finding of a slow shunt in the right temporal pole (dotted arrow).
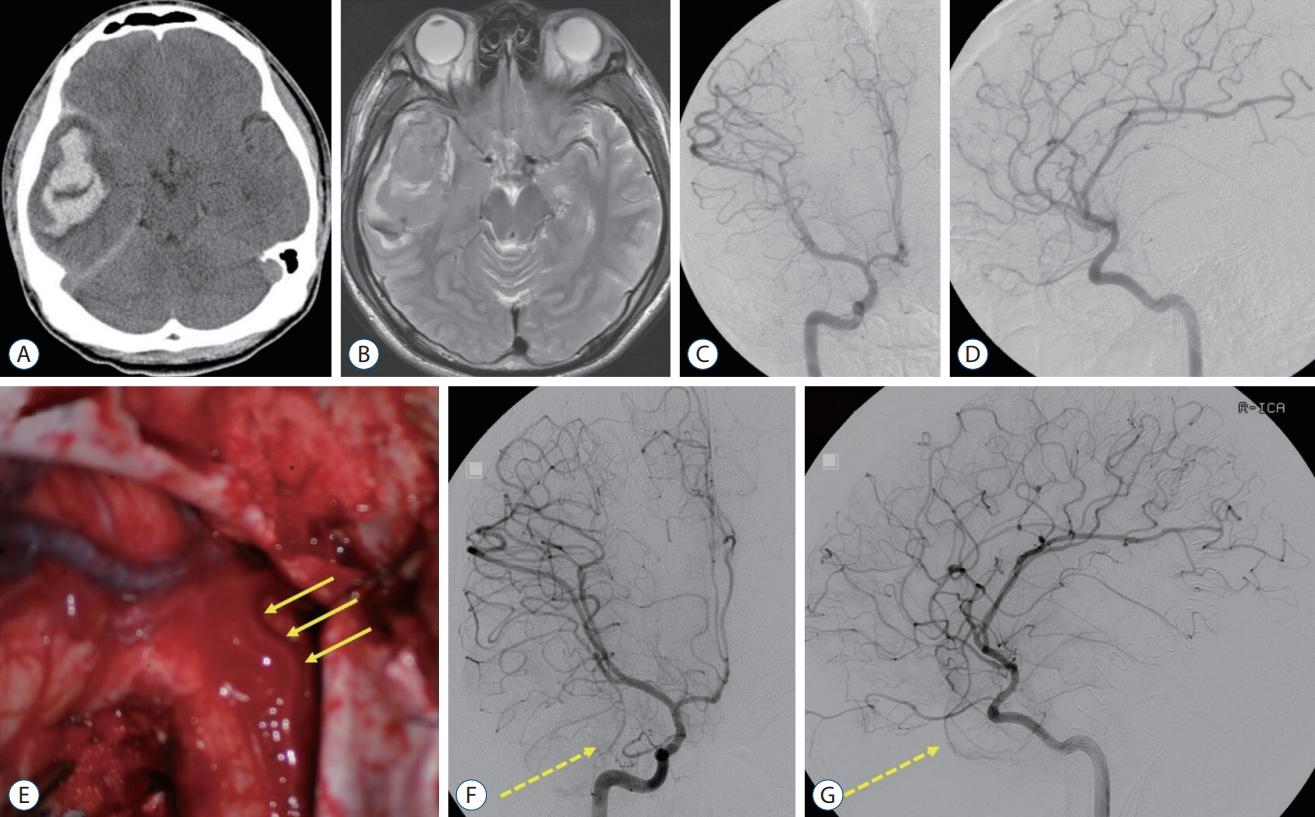
A : There were no abnormal findings except cerebromalatic change in the right temporal lobe on the follow-up brain magnetic resonance (MR) imaging in 1 year after hematoma evacuation. B : On the 11-year follow-up MR imaging, multiple signal voids and enlarged vessels were identified in the right temporal lobe. C-E : Arteriovenous malformation (AVM) was found on digital subtraction angiography (DSA), fed by a few branches of the middle cerebral artery and middle meningeal artery. F : Presurgical glue embolization was partially performed through the anterior temporal artery (arrow). G : Intraoperative photo showed embolized anterior temporal artery by the glue (arrow). H and I : Complete removal of the temporal AVM was confirmed on the postoperative DSA.
In 91 months after the initial surgery, the patient revisited, presenting with recurrent seizures. On a brain MR imaging, multiple signal voids and the enlarged vessels were shown in the right anterior temporal area. A DSA showed a 3.4-cm sized AVM of the Spetzler-Martin grade 2, fed by multiple branches from the right middle and posterior cerebral arteries, and middle meningeal arteries, with the venous drainage into the vein of Labbe and cavernous sinus via the right superficial sylvian vein (Fig. 2). Presurgical glue embolization with Onyx® (ev3, Irvine, CA, USA) was partially performed 1 day before surgery. On the next day, removal of the AVM and right anterior temporal lobe were performed (Fig. 2). The patient was discharged with no additional complication, maintaining the antiepileptic drugs.
DISCUSSION
The annual incidence of ICHs in young adults (<50 years of age) is known about 5 per 100000 individuals [12]. Hypertension and vascular malformations are the most common causes of ICHs in young adults [11,14]. AVMs are the leading cause in a third of the patients with ICHs under 40 years of age [23,29]. Therefore, it is important to confirm the underlying lesions through radiological examinations when young adults present with the ICH [21]. Initially in this case, an AVM was suspicious during the surgery, however, it was not identified on the preoperative and postoperative imaging work-up. There were no abnormal structural lesions even on 1-year follow-up brain MR imaging after the surgery. However, the patient visited again with recurrent seizure and an AVM was finally identified on brain MR imaging and DSA in 11 years after the occurrence of ICH.
The AVMs are intermingled into the brain parenchyme, consisting of arteries, nidus and draining veins with no capillary beds [3]. Diagnosis of the AVMs is usually made with the aid of radiological tools such as brain CT, MR imaging and DSA. However, there have been rare cases diagnosed only by the pathologic examinations after the surgical acquisition of the specimen, with no radiological evidences of the AVMs [2,5,6,20,22,24,26,27,32]. In the pre-MR era when lesions were evaluated with brain CT and DSA alone, such rare cases were called angiographically occult AVMs [22]. Crawford and Russell [4] firstly used the term “cryptic” for small angiomatous malformation that could not be localized due to hematoma and small size. Lobato et al. [17] analyzed 126 cases of angiographically occult AVMs. The mean age at diagnosis was 31.9 years old. The most common clinical symptom was a seizure in 47% of the patients, followed by neurological deficits in 42% and headache in 35%. Microhemorrhage was accompanied on the histological examinations in 80% of occult AVMs. The possible mechanisms by which angiographically occult AVM occurs include small size, mass effect by the adjacent hematoma, and thrombosis of the AVM [18,22]. In this case, it can be speculated that the AVM was not found on the initial radiological work-up because of the small size, mass effect compressing the nidus or thrombus formation within the feeding arteries. As time went on, the AVM grew and the feeding arteries blocked by the thrombus were recanalized, and finally the AVM could be identifiable on the brain MR imaging and DSA. Reports of the growing AVMs are also rare but well known [1,10,25,31]. If the size of the AVM in our case was not small from the initial hemorrhage, pathological angioarchitecture would have been found in brain MR imaging regardless of the mass effect or intravascular thrombosis.
Real occult AVMs have decreased with the introduction of the brain MR imaging [13]. Typical MR findings of AVMs are enlarged and tortuous flow void lesions on T2-weighted images with hemorrhage, adjacent edema and cerebromalacia [7,21]. In addition, abnormal shunting flow is well demonstrated on MR arterial spin labeling which is used to diagnose the small AVMs and dural arteriovenous fistulas, and to evaluate the treatment outcome and recurrence of them [8,16,30]. However, there are still several reports about the occult AVMs undetected on the brain MR imaging and DSA, including this case (Table 1). So, we renamed these as “radiologically occult AVM”. Mean age of the cases was 34 years and 41% were male. As for clinical symptoms, headache was the most common (53% of the cases), followed by seizure (41%). On the brain CT and MR imaging, intracranial hemorrhage including ICH, subdural hemorrhage and subarachnoid hemorrhage, was found in 65%, and calcification was in 42%. Considering that clinical presentations of the usual AVMs are hemorrhage in 50% and seizures in 20–25% [15], radiologically occult AVMs showed a higher tendency of seizure presentation. All cases were finally confirmed as an AVM on pathologic examination. Thrombotic vessels were identified during the surgery in six cases. The prognosis was good in most of cases.
CONCLUSION
Radiologically occult AVMs are very rare with the development of radiological tools. They usually tend to present with cerebral hemorrhage and seizure. Especially in young patients with the ICHs, the causes of ICHs should be initially investigated in order to prevent from recurrence. Even if there are no abnormal findings in the initial radiological examinations, long-term follow-up is recommendable with the possibility of radiologically occult AVMs in mind.
Notes
Conflicts of interest
Won-Sang Cho has been editorial board of JKNS since September 2021. He was not involved in the review process of this original article. No potential conflict of interest relevant to this article was reported.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Author contributions
Conceptualization : WSC; Data curation : HP, HSK, WSC; Formal analysis : HP, WSC; Methodology : HSK, WSC; Project administration : WSC; Visualization : HP, HSK, WSC; Writing-original draft : HP, WSC; Writing-review & editing : HP, HSK, WSC
Data sharing
None
Preprint
None