Hyperkinetic Rat Model Induced by Optogenetic Parafascicular Nucleus Stimulation
Article information
Abstract
Objective
The parafascicular nucleus (PF) plays important roles in controlling the basal ganglia. It is not well known whether the PF affects the development of abnormal involuntary movements (AIMs). This study was aimed to find a role of the PF in development of AIMs using optogenetic methods in an animal model.
Methods
Fourteen rats were underwent stereotactic operation, in which they were injected with an adeno-associated virus with channelrhodopsin (AAV2-hSyn-ChR2-mCherry) to the lateral one third of the PF. Behavior test was performed with and without optical stimulation 14 days after the injection of the virus. AIM of rat was examined using AIM score. After the behavior test, rat’s brain was carefully extracted and the section was examined using a fluorescence microscope to confirm transfection of the PF.
Results
Of the 14 rats, seven rats displayed evident involuntary abnormal movements. AIM scores were increased significantly after the stimulation compared to those at baseline. In rats with AIMs, mCherry expression was prominent in the PF, while the rats without AIM lacked with the mCherry expression.
Conclusion
AIMs could be reversibly induced by stimulating the PF through an optogenetic method.
INTRODUCTION
The basal ganglia performs critical role in coordination of sensorimotor, limbic and cognitive functions in segregated manners. Its function is controlled by two circuits, i.e., the basal ganglia-thalamocortical loop and the basal ganglia-thalamostriatal loop [5,10]. A lot of studies indicated that the abnormal network activity of the basal ganglia-thalamocortical loop is the major pathophysiology of abnormal involuntary movements (AIMs) [1,7,28]. Meanwhile, the basal ganglia-thalamostriatal loop has not been well understood as a source of AIMs [28].
The centromedian (CM)/parafascicular nucleus (PF) is situated at the center of the thalamus [12]. It participates in the basal ganglia-thalamostriatal loop that composed of the striatum, globus paillidus internus (GPi), CM/PF, and thalamostriatal fiber [12,30,33]. This system is topographically well-organized according to sensorimotor, executive, and affective functions [12,34]. The lateral one third of the CM/PF has been suggested to be a region related to motor control [11,19]. Because activation of the basal ganglia-thalamostriatal loop reinforces by itself, the author expected that selective activation of lateral one third of the CM/PF may evoke motor function.
AIM includes several scopes of hyperkinetic movement disorders, such as dystonia, levodopa-induced dyskinesia, tardive dyskinesia, and Huntington’s chorea [16,21,27]. These hyperkinetic disorders characterized by involuntary contractions of skeletal muscles causing stereotypic body movements or abnormal postures [38]. The primary treatment for dystonia is medication, such as γ-aminobutyric acid (GABA) agonists and central-acting anticholinergics. Deep brain stimulation (DBS) of the GPi is a surgical option for treating medically intractable AIMs, especially for dystonia and levodopa-induced dyskinesia [15,39]. There are many animal models related to AIMs, such as DYT mutation models, and psychiatric drug-induced dyskinesia models [16]. To the best of our knowledge, however, there was no study that considers the CM/PF as a potential generator of AIM [21].
The authors hypothesized that AIM can be evoked by artificial stimulation of the PF nucleus of rats, which is the analogous structure of the CM/PF of humans. Here, AIMs resembling dystonia or dyskinesia induced by optogenetical activation of the PF will be introduced.
MATERIALS AND METHODS
All animal experiments were conducted under the approval of the Institutional Animal Care and Use Committee of Soonchunhyang University Bucheon Hospital.
Animal preparation
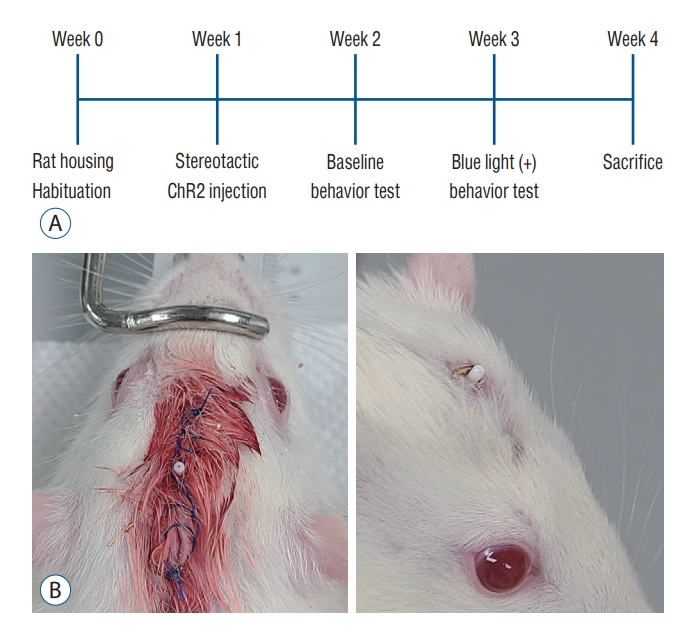
The study subjects were 14 male Sprague-Dawley rats weighing between 200 g and 230 g (Koatech, Pyeongteak, Korea). The animals were housed in an air conditioned room (temperature, ±24°C; humidity, ±60%) under a regular 12-hour light/dark cycle with laboratory pellets and fresh water ad libitum. The housing was maintained 7−10 days before the stereotactic operation. The study protocol is summarized in Fig. 1A.
Stereotactic operation
Rats were anesthetized using Zoletil 50 (Virbac Lab, Carros, France) 40 mg/kg i.p. injection. To achieve accurate targeting to the PF, the author used rat stereotaxic frame (RWD Life Science, Shenzhen, China) for fixation of skull of subject animals. Level of ear bars and incisor bar were set at 30 mm and 27 mm, respectively, to approach the target uniformly. After a linear scalp incision, a burr hole was made for injection of the viral vector and implantation of an optic cannula. A 3.5-µL virus vector conveying channelrhodopsin, AAV2-hSyn-hChR2-mCherry (gene concentration of 2.0×1013 GC/µL; Korea Institute of Science and Technology, Seoul, Korea), was injected using a Hamilton syringe at an injection rate of 0.5 µL/min. The target was the lateral one third of the PF (posterior 4.15 mm, lateral 1.2 mm, and ventral 5.7 mm to the bregma). A fiber optic cannula was stereotactically implanted at the same target, and was fixed on the skull using dental resins. The scalp was closed leaving a small skin opening for exposure of the ceramic ferrule of the optic cannula (Fig. 1B).
Behavior tests
To avoid excessive handling of the subjects while providing an additional measure of locomotor activity, the following three behavior tests were evaluated : AIM score, exploratory behavior, spontaneous alternating behavior (SAB), and lateralization score. The baseline and stimulation behavior tests were performed 7−10 days and 14−17 days after the stereotactic operation, respectively. Details of the tests are described as follows.
Open-field cage test
A subject was placed in a custom-made open-field cage (acrylic plastic, 60×60×40 cm), and was allowed to move freely for 5 minutes with continuous video recording. The AIM score and time of exploration behavior were measured in open-field cage. Exploratory behavior including resting was considered normal. Unnatural movement of the rat such as stereotypic neck motions, rotation of the body, and flexion or extension of limbs were considered AIMs. The AIM score, originally developed to examine levodopa-induced dyskinesia [41], was used to evaluate the degree of dyskinesia. The AIM score was composed of the duration score and three-amplitude score, i.e., axial dystonia, forelimb dyskinesia, and orolingual dyskinesia, and the total score ranged from 0 to 40. Scorings were performed by two independent observers, i.e., observer 1 (M.C.) and observer 2 (a third-party nurse trained for patients with movement disorders).
Y-Maze test
The SAB and lateralization scores were measured in the Y-maze (45×10×25 cm, in length×width×height, respectively), while the rats were allowed to move freely for 5 minutes. A successful alternation was determined when a subject entered three different set of arms in a succession, such as ABC, BCA, CBA, and etc. A ratio of the number of successful alternations to the number of total chances of alternation was considered the SAB score.
For the lateralization score, whether the rat was moving toward the right or to the left at the intersection was counted. The ratio of the number of turns to the right side to the number of total turns was defined as the lateralization score. Because the rats underwent a unilateral surgery on the PF on the left side, it was expected that the optical stimulation may produce increased or decreased movements in a lateralized fashion.
Optical stimulation
The same behavior tests were also performed with optical stimulation >14 days after the injection of the virus. A blue light laser was used to stimulate the target. The laser output was modulated using a function waveform generator (Keysight Technologies, Santa Rosa, CA, USA) connected to the laser diode via a power supply (Model ADR-700A; Shanghai Laser & Optics Century Co., Shanghai, China). For proper delivery of the light while allowing the animals to move freely, a commutator (RWD Life Science) was connected to the optic fiber. The laser output measured at the end of the optic fiber was between 10 mW and 20 mW. Four types of stimulation frequencies were used : phasic stimulation (200−500 mHz), low-frequency (5−12 Hz), intermediate frequency (60 Hz), and high frequency (100−120 Hz) tonic stimulation.
Histologic examination
After i.p. injection of Zoletil 50 (Virbac Lab), intracardial perfusion with 200 mL of ice-cold saline followed by 200 mL of 4% PFA was performed. The rat’s brain was carefully extracted and was steeped in 25% sucrose PBS for cryoprotection for >48 hours. Then, 50-µm thick coronal section were made using a cryotome at -20°C. Every 4th slice was harvested until the appearance of a temporal horn of the lateral ventricle corresponding to the bregma -3.60-mm section in the Paxinos and Watson atlas of the rat brain. Every coronal slice was collected until the emergence of a midbrain contour. Each slice was rinsed twice with phosphate-buffered saline and placed on a glass slide. After the slice was dehydrated, it was coverslipped with a DPX mounting medium (06522; Sigma-Aldrich Korea, Seoul, Korea).
The slide of the section was examined using a fluorescence microscope (Zeiss Axio Observer A1 Inverted Phase Contrast Fluorescence Microscope, Oberkochen, Germany). As a background, a low power field (×10) phase contrast image was taken under simple bright light illumination per specimen. Then, a phase contrast image in a medium power field (×50) was taken, which was followed by acquisition of a paired fluorescence image with an exposure time of 4000 ms to detect mCherry under ultraviolet light. The paired histologic images were fused using a plugin of the ‘Colocalization finder’ in the ImageJ software (National Institutes of Health, Rockville, MD, USA) (Supplementary Fig. 1).
In the brain histology, midline, outer border of the thalamus, and lateral and third ventricles were clearly identified and were used to estimate location of the PF. Another prominent structure found in microscopic examinations was the fasciculus retroflexus (fr) that lies within the PF. Using the ‘distance’ tool of the ImageJ, locations of the tip of the fiber optic cannula were measured in distances (mm) from the midline and from the dorsal border of the thalamus. The area of mCherry fluorescence was also measured using the same tool of the ImageJ.
Statistical analysis
SPSS statistics (version 19; IBM Co., Armonk, NY, USA) was used for the statistical analyses. Two observers independently rated the AIM scores, which were averaged (sum of AIM scores / number of observations) to compare the AIM scores of pre- and post-stimulations using pared t-test. To verify reliability of the AIM scores between the two observers, inter-observer reliability was tested using intraclass correlation coefficient. To compare AIM scores among four different stimulation frequencies, one way analysis of variance (ANOVA) was used. Histological findings were compared between rats with dyskinesia and without dyskinesia. For quantitative examination, the area (mm2) of mCherry expression and the location of tip of the fiber optic cannula were compared using the Mann Whitney U test. All analyses were performed using a two-tailed test, and the significance level was defined as p<0.05, unless otherwise specified.
RESULTS
Among the 14 rats, seven rats (rats 1, 4, 8, 9, and 12−14) displayed clear AIMs after blue light stimulation. Two types of AIMs were found; one was hyperkinetic movement of the whole body, and the other was involuntary motion of the cervical region resembling cervical dystonia (Supplementary Video 1).
Ipsilateral rotations with vibrant flexion/extension of body and extremities were found in rats 1, 9, 12, and 13 (Fig. 2). In rat 1, the trunk and neck were bent >90° to the left, and the rats were rotated. The right forelimb moved violently, pushing the right-side of the body away from the floor. The remaining three limbs showed passive movements to compensate the abnormal posture caused by abnormal movements of the right forelimb. Faster intense rotations to the left side were observed in rat 9, although the flexion of the body was rather modest than that in rat 1. Rat 12 showed ipsilateral rotations with a violent flail of the four limbs, which was accompanied by backward stepping movements. Rat 13 showed an involuntary bend of the body to the left side with extension of the right limbs. It resulted in a sway gait and modest rotation movement to the left side.

Abnormal involuntary movements resembling dyskinesia or dystonia. This figure shows representative abnormal involuntary movements (AIMs) observed in the open-field test. Rat numbers are shown in the left column. Before stimulation (baseline, second column images), every rat did not show any abnormal involuntary movement. With blue light optical stimulation (with stimulation, third column images), each rat showed specific AIMs, and schematic illustration of these movements are shown in the right column. Rats 1, 9, 12, and 13 showed AIMs, with the whole body being rotated counter-clockwise (long arrows). Rotation movements were accompanied by abnormal limb motions (short arrows). Rats 4, 8, and 14 showed AIMs restricted to upper body or neck motions (long arrows), which were resembling laterocollis, torticollis, and anterocollis, respectively.
AIMs restricted to the neck portion were found in rats 4, 8, and 14, resembling cervical dystonia (Fig. 2). In rat 4, the optical stimulation induced right-sided tilting of neck that may have been laterocollis. The blue light laser immediately evoked the involuntary neck tilting, although there was a time delay until reaching the maximum degree of neck tilting. Thereafter, the abnormal posture was diminished, which the rat was believed to be tolerated to the constant optical stimulation. Rat 8 showed neck rotations resembling torticollis. Phasic stimulation (200−500 mHz) induced sustained phasic head rotation to the right side. In contrast, tonic stimulation (12−120 Hz) caused a strong head rotation in an earlier period, which was followed by stimulation tolerance that was shown in the rat 4. In rat 14, the optical stimulation caused involuntary ventral flexion of the head and body, which mimicked anterocollis.
In the other seven rats (rats 2, 3, 5−7, and 10), no apparent AIMs were observed. The animals exhibited only spontaneous behaviors irrespective of optical stimulation during the whole video recordings. Optogenetic stimulation test could not be done in rat 5 due to a surgical wound infection and expulsion of the optic cannula.
Quantitative measurement of the behavior test
The AIM scores are shown in Table 1. Before the stimulation, no AIM was found, so that all baseline AIMs were scored zero. After light stimulation, the average AIM score was increased to 9.9±11.8, which was significant (paired t-test, p=0.011; Fig. 3A). Seven rats (rats 1, 4, 8, 9, and 12−14) presented with stimulation-induced AIMs were classified as the ‘AIM’ group. The other seven rats without AIM (rats 2, 3, 5−7, 10, and 11) were classified as the ‘No AIM’ group.
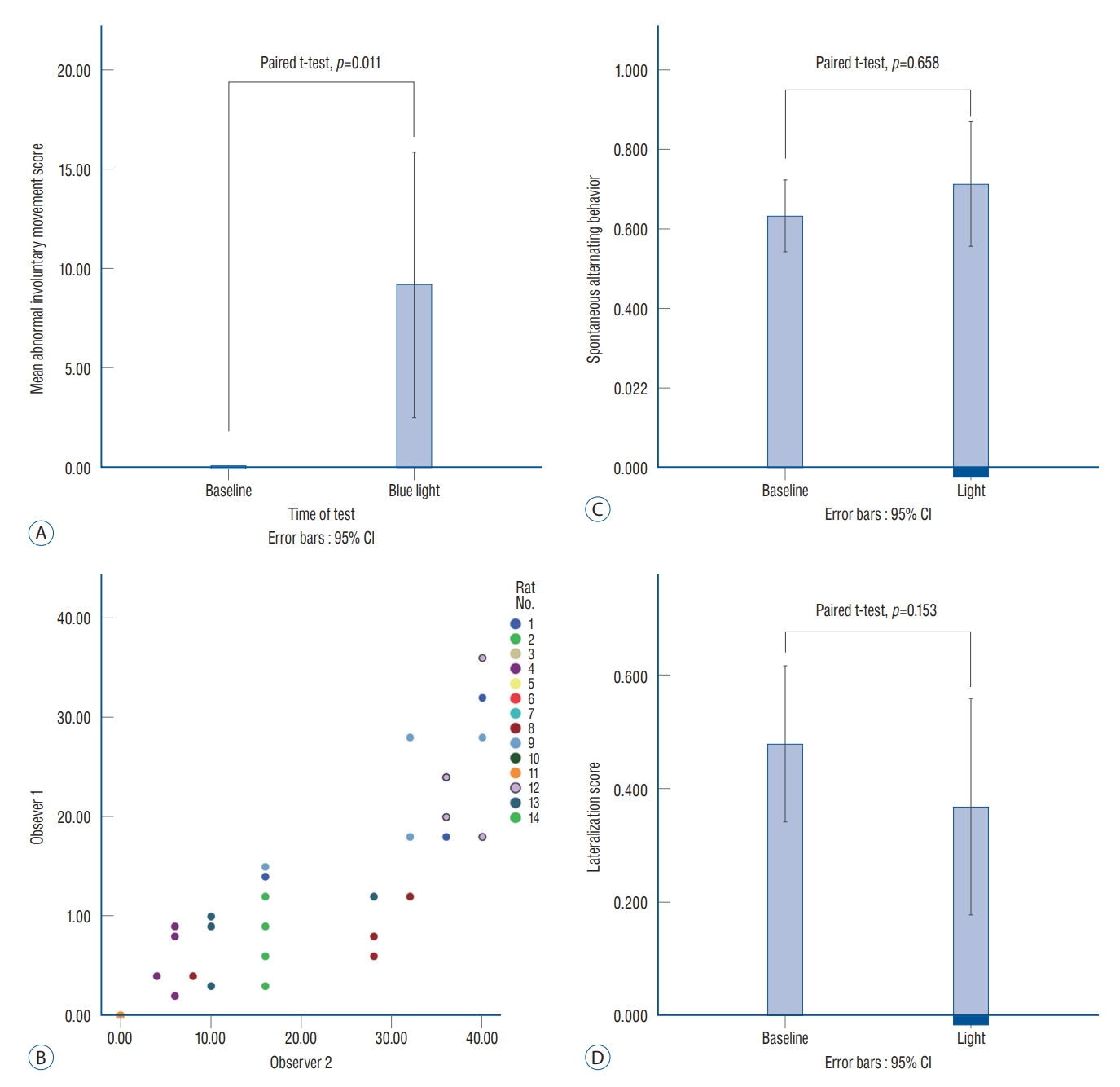
Statistical analyses of behavior tests. After blue light stimulation, the average abnormal involuntary movement (AIM) score was significantly increased compared to the baseline (A). The AIM scores rated by the two individual observers (observers 1 and 2) showed a significant correlation (B). Spontaneous alternating behavior and lateralization score were not significantly changed after the light stimulation (C and d). CI : confidence interval.
The AIM score was measured differently as the stimulation frequency was changed from 0.2 Hz to 120 Hz, although it was not significant (one way ANOVA, p=0.326). The intraclass correlation coefficient showed significant reliability (correlation coefficient=0.914, p<0.0001; Fig. 3B) between the two individual observers (observers 1 and 2).
The SAB scores were 0.63±0.15 and 0.71±0.26 before and after optical stimulation, respectively (paired t-test, p=0.658; Fig. 3C). The lateralization scores decreased after blue light stimulation and were 0.48±0.22 and 0.37±0.32 at the baseline and after optical stimulation, respectively (paired t-test, p=0.153; Fig. 3D). The authors found a tendency in some rats to choose the left direction after stimulation. This change may have occurred not because the rats preferred the left side by stimulation, but because the rats had involuntary movement to turn their body to the left side (Supplementary Fig. 2).
Histologic examination
Visual assessment revealed that strong expressions of the mCherry encompassing the PF were frequently found in many brain sections of the rats in the AIM group (Fig. 4). In contrast, only a subtle mCherry expression was found in the PF of the three brain sections in the No AIM group.

Histological findings of the rats. Rats with abnormal involuntary movements (AIMs) (the left column, rats 1, 4, 8, 9, and 12−14) exhibited prominent expression of the mCherry encompassing the parafascicular (PF) nucleus. In contrast, rats without abnormal involuntary movement (the right column; rats 2, 3, 5−7, 10, and 11) rarely presented the mCherry expressions.
In the quantitative analyses, significant differences were observed in the area of the mCherry expression, which were 2.95±0.77 mm2 and 0.94±0.64 mm2 for the AIM and No AIM groups, respectively (Mann-Whitney U test, p=0.002; Table 2). The average lateral and ventral distances of the tips were 1.57± 0.37 mm and 1.85±0.75 mm, respectively. By matching the coordinate of the Paxinos and Watson atlas [25], the tip was found to be located at the lateral one third of the PF that is the exact location the the authors initially intended (Fig. 5A). Optic cannula tip locations of each rat were plotted on the lateral distance (mm) and depth (mm) plane (Fig. 5B). Despite no significant difference in the averaged values (Mann-Whitney U test, p=0.902), the visual inspection showed that the tips of the AIM group were clustered together, while the tips of the No AIM group were scattered from each other.
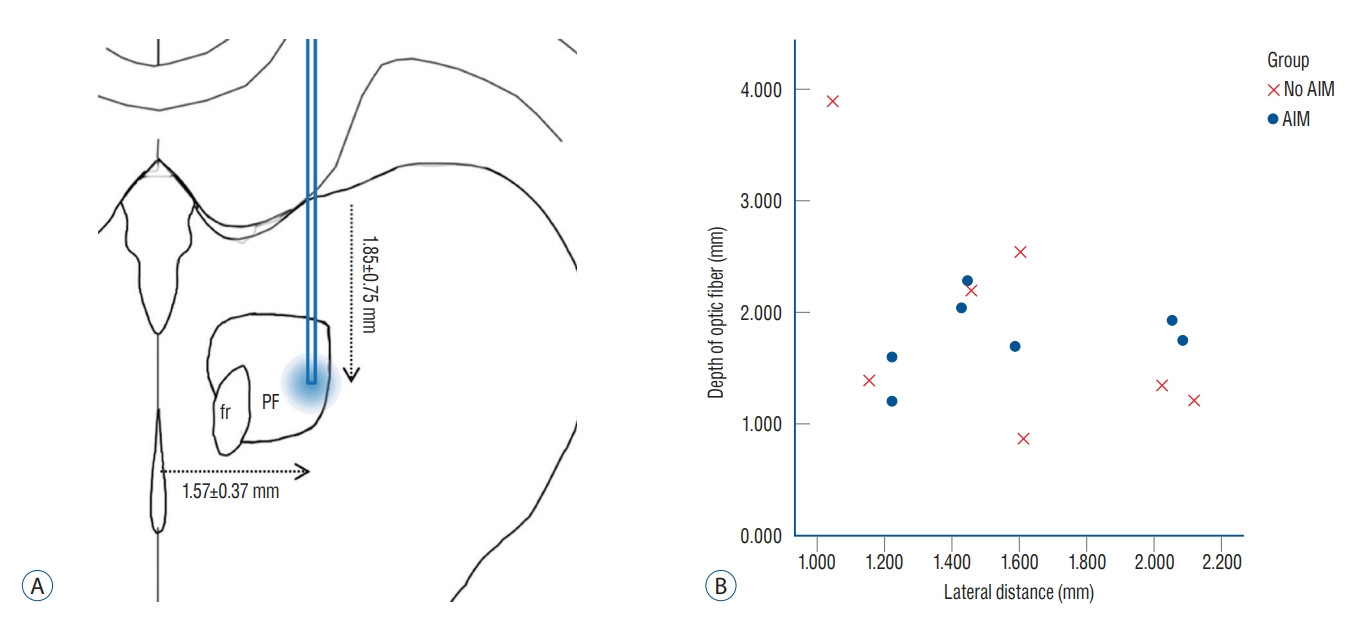
Location of tip of the optic cannula. The averaged location of the tips was illustrated with reference with the Paxinos and Watson atlas (A). The tip was found to be located at the lateral one third of the PF. Optic cannula tip locations of each rat were plotted on the lateral distance (mm) and depth (mm) plane (B). fr : fasciculus retroflexus, PF : parafascicular nucleus, AIM : abnormal involuntary movement.
DISCUSSION
This study showed that AIMs could be artificially generated when the PF was stimulated optogenetically. Anatomically, the CM/PF in humans and the PF in rodent species project glutamatergic fibers to the striatal medium spiny neuron (MSN) [24]. The MSN also receives other modulatory inputs such as dopamine, acetylcholine, serotonin, and GABA, but the glutamatergic input from the thalamostriatal afferent has been regarded as the most potent signal that modulates striatum (Fig. 6). Three functional regions of the PF, i.e., sensorimotor, associative, and limbic regions, are topographically well-organized from lateral to medial distribution, respectively [18]. The lateral one third of the PF is known as the sensorimotor region [18].
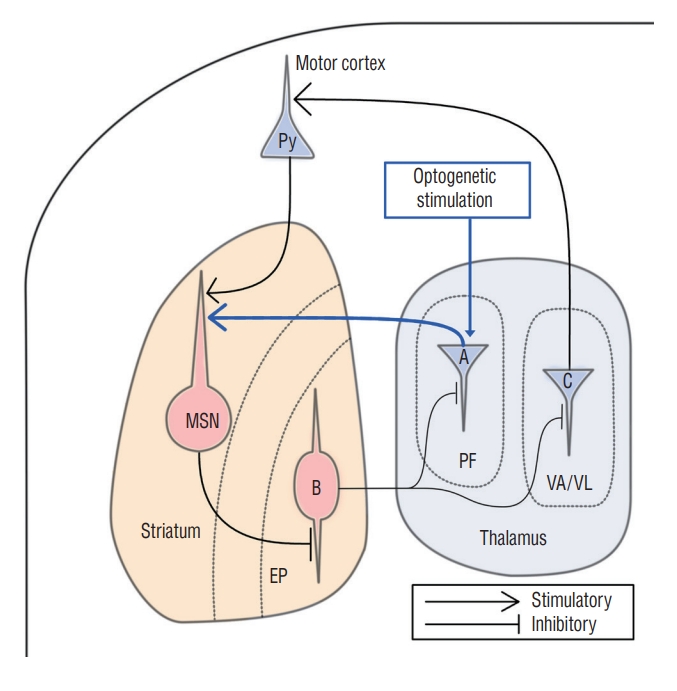
Schematic illustration of relevant brain networks. The striatal medium spiny neuron (MSN) receives two major inputs from the pyramidal cell (Py) of the cerebral cortex and the parafascicular nucleus (PF). A driver neuron (A) of the PF sends glutamatergic efferent to the MSN, called thalamostriatal circuit that provides a potent stimulatory signal to the MSN. The MSN relays γ-aminobutyric acid (GABA) signal to entopeduncular nucleus (EP), the homologous structure of globus pallidus internus in the human. A projector neuron (B) of the EP releases GABAergic efferent to the PF and the ventral lateral/ventral anterior (VA/VL). A driver neuron (C) of the VA/VL relays glutamatergic signal to the motor cortex, activating the basal ganglia−thalamocortical loop. Optogenetic stimulation of the PF (blue box) may increase glutamatergic signal to the MSN (blue arrow). It activates the MSN followed by facilitation of the basal ganglia-thalamocortical loop, which will induce abnormal involuntary movements.
Here, the histologic examinations showed a strong expression of the mCherry encompassing the PF in the rats with dyskinesia, and the optic cannula tips were found approximately at one third of the PF. This indicates that proper transfection of the virus at the intended target was the key factor for successful manifestations of AIMs. Therefore, it can be said that the optogenetic stimulations of the lateral one third of PF caused AIMs resembling dystonia or dyskinesia. Activation of the sensorimotor region of the PF might increase the glutamatergic output via the thalamostriatal circuit, followed by activation of the reinforcement loop, which have caused hyperkinetic movements.
Even though half of subject animals showed expected results, it also should be addressed about negative results in other half of rats. The authors thought that primary reason of the failure was inappropriate virus transfection, because mCherry expression were not apparent in brains of rats with negative results (Fig. 5A). Due to technical problems, the time to carry virus to the target brain region after unfreezing the virus in room temperature was too long in rats 2, 3, 6, 7, 10, and 11 at the time of experiment. In rat 5, post-operative wound infection might be principal cause of a negative result. Furthermore, vertical and lateral distances of the optic cannula tip from the ventricular walls were more scattered in negatively resulted cases (Fig. 5B). It indicates that technical issues such as the lack of a surgical microscope in the animal experiment could influence these errors in inducing AIM.
It is worthy to compare the yield rate of the AIM 50% in this study to that success rates obtained by other animal models of AIM in other literatures. The most well known animal model of dystonia is DYT1 gene mutation mode [l3]. Because gene mutation model dose focus on etiological factor rather than abnormal movement itself, presentations of AIM were not apparently addressed in the literatures [21]. In animal model with levodopa induced dyskinesia produced by 6-OHDA injection, success rates of dyskinesia presentation were reported to be between 63% and 100% according to injection dose of 6-OHDA [26]. In another animal study examined amphetamine induced abnormal movement and AIM induced by levodopa, 120 of 312 (38.5%) rats were reported to show dyskinesia [36].
In contrast to traditional animal models of movement disorders, optogenetic animal model has several advantages. With optogenetic techniques, a researcher can stimulate or inhibit neurons of a specific cell type in a specific anatomical region. Moreover, this neuromodulation can be done with tremendous temporal precision [17]. Especially, when optogenetic techniques combined with genetic engineering technique that changes specific gene expression of subject animals, super-selective control of specific neurons or circuits becomes possible [9]. This potential advantage of optogenetics prompted the author to choose optogenetic tool to make an animal model of AIM.
Because mCherry was also found in adjacent thalamic structures, such as the posterior thalamic nuclear group and the parvicellular part of the ventral posterior nucleus, unintentional activation of these structures should be considered [25]. Posterior thalamic nuclear group has a role in sensory information processing especially related with selective attention [6]. It also sends the glutamatergic efferent to the striatal MSN less abundantly than the PF [24]. Therefore, it might be suspected that the posterior thalamic nuclear group also has a role in development of AIM in our rats. The parvicellular part of the ventral posterior nucleus participates in gustatory function of the thalamus [31], which appears to be less likely to induce AIM.
There are well-known patterns found in the microelectrode recordings during GPi DBS in patients with dystonia and Parkinson’s disease [2,4]. Repeated bursting spike activities are characteristic of patients with dystonia, whereas continuous tonic firing patterns are observed in patients with Parkinson’s disease [20]. In a human autopsy study, the CM/PF has been demonstrated to be degenerated in Parkinson’s disease and Huntington’s disease [42]. Moreover, intractable freezing of gait in Parkinson’s disease was improved by DBS of the CM/PF [19]. In a mouse model of late-stage Parkinson's disease, significant alterations in the functional connectivity of the PF with the striatum was found to contribute to motor deficits associated with the disease [13,19,40]. Other researchers have reported that an imbalance arises in the responsiveness of the PF stimulation, which was mediated by cholinergic interneuron [35]. However, the precise mechanism behind motor control of the PF and its functional relevance continues to evolve.
The CM/PF also participates in several important brain functions rather than sensorimotor control, i.e., arousal, executive function, and affective pain processing [32,34]. The CM/PF receives cholinergic input from the brain stem, which is relayed to vast swaths of the cerebral cortex [12]. The PF also has strong reciprocal glutamatergic connections to the prefrontal cortex and the anterior cingulate cortex, which participates in executive function with a feedback loop between the basal ganglia and the CM/PF [6,14,22]. Suppression of the CM/PF compromises not only motor function but also executive control function as well [11,23,29,31,37]. The PF receives pain and temperature sensory information, which is in turn connected to the anterior cingulate cortex [8]. Within this structure, the CM/PF is considered to participate in the processing of affective pain response and polysensory integration [32,40].
This study has several limitations. Most importantly, there was no predefined control group. The reason why the author did not make predefined control group was that very obvious AIMs were found during optical stimulation. The author determined that it would be more meaningful to compare rats with and without AIM than to create a predefined control group. The author also found several types of AIMs in this study, although it is ambiguous as to which factor affected the variance of the phenotypic diversities. Moreover, substantial success rate in this study should be improved, which may be achieved by applying precise animal experiment tools or use of genetic engineering technique. It would lead to a discovery of exact role of specific nuclei in the generation of hyperkinetic movements in future studies.
CONCLUSION
Optogenetic stimulation of the PF successfully induced hyperkinetic movements in the rat model. This study presents a noble concept that the PF may play an important role in the development of hyperkinetic.
Notes
Conflicts of interest
Moonyoung Chung has been editorial board of JKNS since November 2017. He was not involved in the review process of this article. No potential conflict of interest relevant to this article was reported.
Informed consent
This type of study does not require informed consent.
Author contributions
Conceptualization : MC; Data curation : ; Formal analysis : MC; Funding acquisition : MC; Methodology : MC, YSP; Project administration : MC; Visualization : ; Writing - original draft : MC, YSP; Writing - review & editing : MC, YSP
Data sharing
None
Preprint
None
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant, funded by the Korean Ministry of Science and ICT (NRF-2019R1G1A1100439). This work was also supported by the Soonchunhyang University Research Fund.
This work is a reuse of part of a thesis; Moonyoung Chung, August 2021, ‘Optogenetic parafascicular nucleus Stimulation Induced Hyperkinetic Rat Model’, PhD thesis, Chungbuk National University, Cheongju, Korea.
Supplementary materials
The online-only data supplement is available with this article at https://doi.org/10.3340/jkns.2022.0106..
Colocalization of histologic images using ImageJ processor. A phase-contrast image was obtained in an area around the thalamus (A). Then, a paired fluorescence image was obtained at the same location as the background phase-contrast image (B). Two paired images were localized using the ‘Colocalization finder’ tool in the ImageJ software. The final product was composed of a green background and red fluorescence (C).
A left-side turn caused by the light stimulation. This figure shows how the rat ended up choosing the left direction. The rat was searching in the right direction at the intersection of the Y-maze, but the light stimulation forced its body to the left side.
Essential video clip of abnormal involuntary movements. This video shows abnormal movements resembling dyskinesia or dystonia of the rats. The word “ON” on the screen indicates that optogenetic stimulation is being applied. Without the stimulation rats showed natural behavior such as searching environments or resting on the ground. With the stimulation, each rat exhibits characteristic abnormal involuntary movements such as body rotation, abnormal step, neck tilting, and neck flexion.