Characteristics of Focused Ultrasound Mediated Blood-Brain Barrier Opening in Magnetic Resonance Images
Article information
Abstract
Objective
The blood-brain barrier (BBB) is an obstacle for molecules to pass through from blood to the brain. Focused ultrasound is a new method which temporarily opens the BBB, which makes pharmaceutical delivery or removal of neurodegenerative proteins possible. This study was demonstrated to review our BBB opening procedure with magnetic resonance guided images and find specific patterns in the BBB opening.
Methods
In this study, we reviewed the procedures and results of two clinical studies on BBB opening using focused ultrasound regarding its safety and clinical efficacy. Magnetic resonance images were also reviewed to discover any specific findings.
Results
Two clinical trials showed clinical benefits. All clinical trials demonstrated safe BBB opening, with no specific side effects. Magnetic resonance imaging showed temporary T1 contrast enhancement in the sonication area, verifying the BBB opening. Several low-signal intensity spots were observed in the T2 susceptibility-weighted angiography images, which were also reversible and temporary. Although these spots can be considered as microbleeding, evidence suggests these are not ordinary microbleeding but an indicator for adequate BBB opening.
Conclusion
Magnetic resonance images proved safe and efficient BBB opening in humans, using focused ultrasound.
INTRODUCTION
The blood-brain barrier (BBB) is a specialized structure in the brain consisting of brain-specific endothelial cells, basement membrane, pericytes, and astrocytic end feet [1,8]. The BBB separates the circulation of the blood and cerebrospinal fluid in the central nervous system (CNS) by forming a closed membrane boundary around all CNS capillaries; this membrane does not exist in extra-cranial circulation [35]. Brain endothelial cells are linked via tight junctions [34], which limits paracellular transportation. Furthermore, the lack of fenestrations and transport vesicles limits transcellular transportation and prevents crossing of large molecules [5]. This prevents the spread of large molecules or microorganisms into the cerebrospinal fluid [33]. Therefore, the BBB is critical for maintaining homeostasis and protecting the CNS from toxic materials [33-35]. Nevertheless, the presence of the BBB limits the transportation of drugs and poses a significant obstacle to the delivery of pharmaceutical drugs for treating many brain diseases, such as glioblastoma (GBM) and Alzheimer’s disease (AD) [5,33,35].
Developing methods for facilitating transport pathways to enhance drug delivery and clearing of neurotoxic materials are an active field of research [5,13,33,35]. Several techniques have been used to bypass the BBB [33]. Invasive techniques, such as direct injections, can be employed, but surgical interventions are associated with substantial risks and complications [33]. Furthermore, the infiltration of the agents into the parenchyma may be limited by diffusion [33]. Therefore, noninvasive techniques are actively being developed [5,13,33]. Currently employed techniques include the use of carrier proteins, pharmacological modifications, virus-mediated delivery, exosome-mediated delivery, intranasal delivery, and BBB permeability modulation, using osmotic agents or focused ultrasound (FUS) [33].
Magnetic resonance (MR)-guided FUS (MRgFUS) surgery is a novel technique that uses ultrasound acoustic energy to treat intracranial disease. The US Food and Drug Administration approved MRgFUS thalamotomy as a treatment for medication-refractory essential tremor in 2016, and ablative MRgFUS is now being investigated for the treatment of various neurological diseases, such as movement disorders [7,10,24,31], psychiatric disease [6,18,20] and brain tumors [26].
Low-intensity FUS is used to open the BBB transiently and reversibly in the target regions [4,15,25]. Hynynen et al. modified the low-intensity FUS method to produce safe and reproducible BBB openings using microbubbles [14-16]. Extensive research on BBB opening for drug delivery is underway [5,33,40]. Indeed, transient BBB opening in target brain regions can facilitate drug delivery for treating CNS diseases as well as neurodegenerative disorders, given the potential for promoting the clearance of neurotoxic proteins or particles in neurodegenerative disorders, such as GBM and AD [36,38]. Recently, we reported preliminary results of MRgFUS-mediated BBB opening combined with intravenous injection of microbubbles in patients with AD and GBM [36-38].
In this study, we further analyzed the MR findings as a consequence of multiple MRgFUS induced BBB openings in patients with AD and GBM. Additionally, we aimed to evaluate the clinical safety of MRgFUS-induced BBB opening using MR findings during the entire treatment period.
MATERIALS AND METHODS
Patients
This retrospective study included a review of two prospective clinical trials (AD clinical trial registration No. : NCT04526262 [clinical trials.gov], GBM temozolomide [GBMTMZ], clinical trial registration No. : NCT03712293 [clinicaltrials.gov]) that were also approved by the Institutional Review Board of Severance Hospital (IRB No. 1-2018-0040 and 1-2019-0095). Eleven patients were included in this study. Six patients (five females and one male; mean age, 67.3±13.9 years) were included, but one dropped out in the AD prospective study. Eight patients were included, but two dropped out in the GBMTMZ study (two females and four males; mean age, 55.8±11.03 years). Patients with AD were all diagnosed with dementia according to the Mini-Mental State Examination (MMSE) scores (scores below 22 in all six patients), and 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) and 18F-florbetaben (FBB) PET showed consistent findings for AD in these patients. Patients with GBM underwent gross total resection of their tumor, which was pathologically confirmed as a World Health Organization grade IV malignant GBM by a neuropathologist. Excluding the main diagnoses, the patients did not have any other history of brain diseases, such as major cerebral infarction or intracranial hemorrhage. They did not have other comorbidities such as significant cardiac disease, uncontrolled hypertension, or bleeding tendency.
MRgFUS procedure
We performed a total of six cycles of BBB opening following the standard Stupp protocol with TMZ chemotherapy for five patients with primary GBM [43]. In the standard TMZ treatment regimen, one cycle was defined as 4 weeks. On the first cycle, 150 mg/m2 of TMZ was taken orally for the first 5 days. On the next 2nd to 6th cycles, a dose of 200 mg/m2 was taken PO as maintenance dosage for the first 5 days of each cycle. Each BBB opening was performed at one-month interval on the 1st or 2nd day of the 4-week chemotherapy protocol. Five patients with AD underwent two cycles of BBB opening with a 3-month interval without additional therapeutics.
BBB opening was performed with a MRgFUS system consisting of 1024 transducer elements with 220 kHz frequency (ExAblate Neuro; InSightec, Haifa, Israel) under continuous infusion of microbubble contrast (Definity®; Lantheus Bedford, MA, USA) (250 mL normal saline + 1.3 mL Definity®, infusion rate 180 mL/h). During the procedure, MR imaging (MRI) was acquired for interim evaluations of the patient, and real-time acoustic signal monitoring was performed to determine the sonication parameters. For imaging guidance, a 3.0-T MR magnet (MRI discovery MR 750; GE Healthcare, Chicago, IL, USA) was used. T1-weighted 3D and T2-weighted images were acquired and co-registered with prediction MR images for precise targeting.
Before the procedure, the patient’s hair was shaved and chemically depilated. A stereotactic frame was fixed to the patient’s head after administering local anesthesia with 1% lidocaine. The stereotactic frame was attached to a helmet-shaped transducer array inside the MRI room. After sonication, the patient was examined for adverse effects.
For the primary GBMTMZ study, the MRgFUS target was set within 2 cm of the resection margin in the white matter with a 1 cm3 sized grid (shape of 3×3, 9 spots) for each sonication. For patients with AD, MRgFUS sonication targets were not limited to a 1 cm3 sized grid. Due to the updated version of the device, larger BBB opening per one sonication was available. We attempted to cover an extensive area in the white matter of both frontal lobes for patients with AD (Fig. 1).
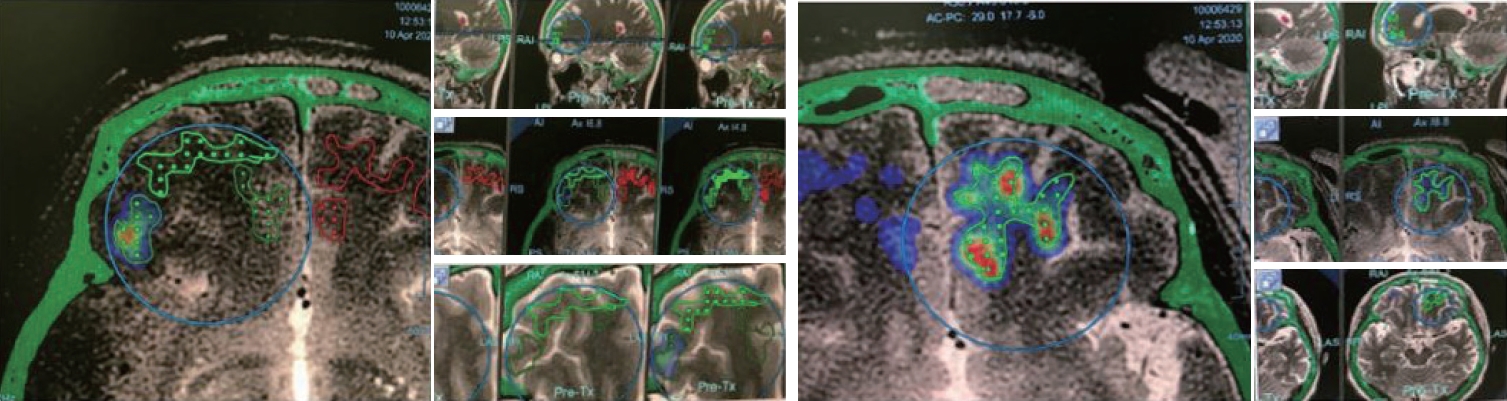
Planning of blood-brain barrier opening area in Alzheimer’s disease study with focused ultrasound device. Area of grid with dots is the planned area for blood-brain barrier (BBB) opening. Area of BBB opening can be monitored in a heatmap shape by realtime acoustic emission monitoring.
To minimize the risk, we avoided areas containing sulci and vessels. To avoid overlap sonication we gave 0.8–1.0 cm intervals in sagittal planes in each sonication (Fig. 1). After setting the target, microbubble contrast was injected, followed by the application of low-frequency FUS to the target. Real-time MR thermometry was performed to monitor tissue temperature during sonication. The area of BBB opening was detected using the real-time acoustic emissions monitoring (AEM) technologies measuring the oscillation of microbubbles and feedback loop system was used to control the threshold of FUS effects.
Each target power ramp test was performed after the injection of microbubbles. Using the power ramp test, we determined the optimal parameters for a safe BBB opening. We started with a power of 8 W and gradually ramped up the power until the accumulated cavitation dose reached the target of 0.4–0.65 MPa, up to a maximum power of 40 W. Sonication was performed for 90 seconds per session. When inertial cavitation was detected, sonication was stopped immediately. In the case where a sufficient dose was not reached despite a sufficiently high power, 120 seconds long sonication was used. Several sonication procedures were performed to include the entire target area. By recording and measuring the AEM data during bbb opening, cumulated acoustic cavitation data was recorded in real-time and used to calculate cavitation dose shown as heatmaps (Fig. 1). After the completion of the procedure, gadolinium contrast-enhanced MRI was performed to verify whether BBB opening was successful. Then, additional high-resolution MRI sequences were obtained from the patient after removing the FUS device and stereotactic frame.
MRI techniques
All patients with GBM underwent MRI evaluation within 1 week before treatment and immediately after each of the six BBB opening cycles. Patients with AD underwent baseline brain MRI within a month before treatment, and immediate posttreatment brain MRI was acquired after completion of each session. A follow-up brain MRI was also performed 2 months after the completion of the treatment. All MR scans were acquired using a 3T MR system (MRI discovery MR 750; GE Healthcare) with a 32-channel sensitivity-encoding head coil.
The MRI protocol included axial 2D T1-and T2-weighted imaging fast spin echo, 3D fluid-attenuated inversion recovery, susceptibility-weighted angiography (SWAN) imaging, diffusion-weighted imaging, 3D spoiled gradient echo T1WI, and contrast-enhanced 3D spoiled gradient echo T1WI. Contrast enhanced images were obtained after administration of a Gadolinium-based contrast agent (Gadovist; Bayer, Toronto, Canada) at a dose of 0.1 mL/kg.
MR analysis was independently conducted by neuroradiologists and reviewed by neurosurgeons. Serial post-treatment MRIs were compared with the baseline MRI data to assess the presence of parenchymal and extraparenchymal enhancement, location of parenchymal enhancement, parenchymal and extraparenchymal hemorrhage, T2 and diffusion signal changes of the targeted area, and mass effect.
RESULTS
The clinical outcome and safety of MRgFUS induced BBB opening procedures
No procedure-related neurological complications were observed in any of the BBB opening studies. The patients’ main concern with MRgFUS induced BBB opening was the discomfort and pain caused by stereotactic frame fixation and repeated hair shaving.
In the GBMTMZ study with more than 1 year of follow-up, only two patients showed recurrence at 11 and 16 months of follow-up, respectively. The other four patients showed no recurrence for an average of 15 months. All patients responded to treatment such that the tumor size reduced or remained unchanged according to the response assessment in neurooncology (RANO) criteria during follow-up (Fig. 2). The survival rate of up to 13 months was 100%. None of the patients with GBM had immediate or delayed BBB opening related complications.
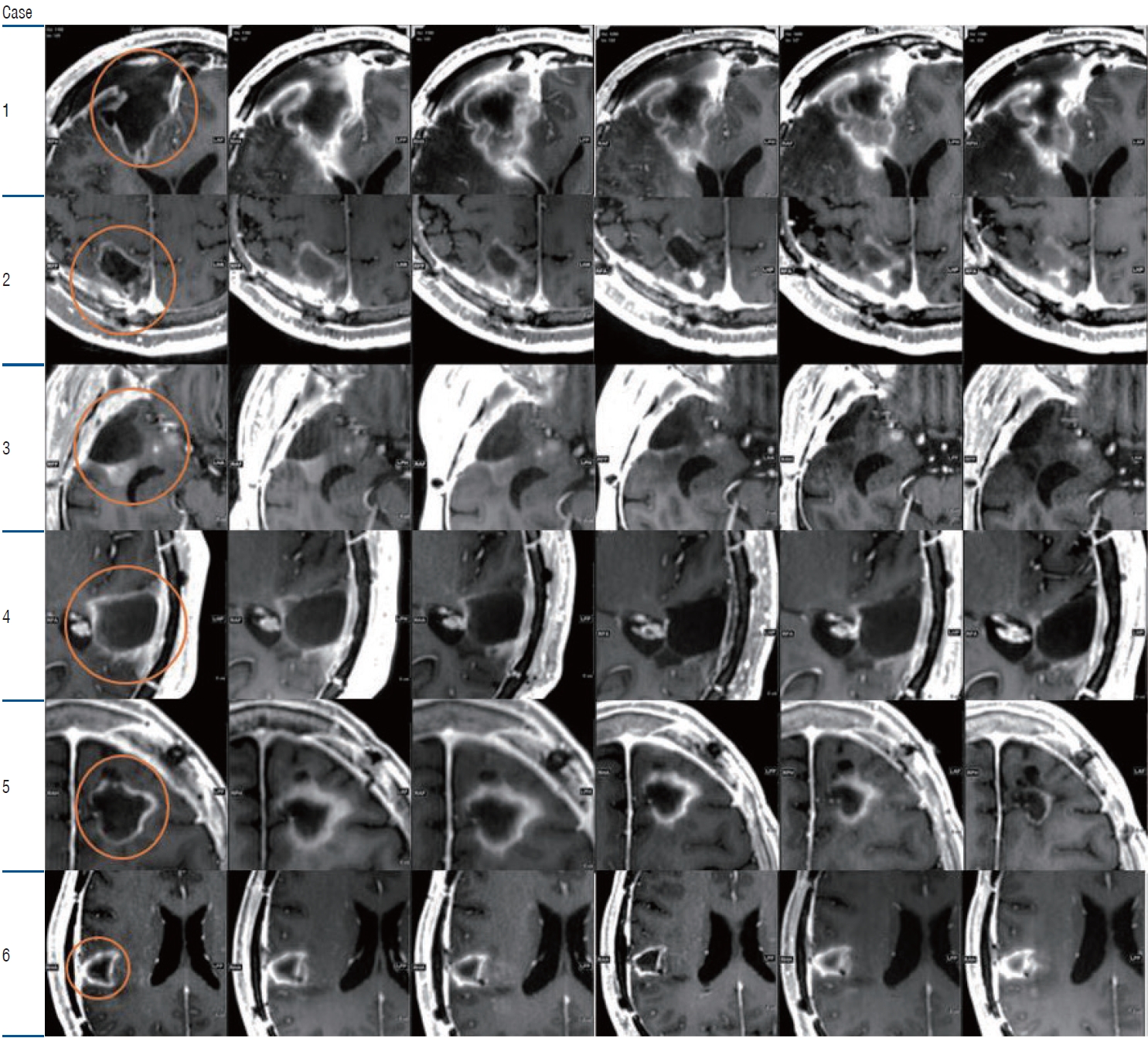
Sequential T1 contrast images of glioblastoma blood-brain barrier opening study. Each row is the follow up magnetic resonance image of each temozolomide cycle. Orange circle indicates the region of interest. The tumor size has been stable or partially reduced during the follow-up sequential images.
For patients with AD, we attempted to open the extensive area in the frontal lobe and observed a reduction in Aβ deposition in both frontal areas treated with 18F-FBB PET.
Even though we observed a change in the signal intensity on MRIs after BBB opening, we did not observe any worsening of neurocognitive function. Instead, we noticed transient improvement in symptoms and neuropsychological test scores such as the Caregiver-Administered Neuropsychiatric Inventory and the Korean version of the MMSE.
MRI analysis
T1 contrast images revealed effective BBB opening through extravasation of contrast agents
In both patients with GBM and AD, BBB opening was observed on T1-weighted contrast images. On T1 contrast images, the BBB opening targets of patients with GBM exhibited a high signal intensity with nine visible spots, which was the shape of a 3×3 grid with a sonication area of 1 cm3. Among the 145 GBMTMZ BBB opening targets, Gd enhancement was observed in 131 targets (90.3%). In the BBB opening of patients with AD, parenchymal enhancement of the frontal lobes was identified for each case using contrast enhanced T1-weighted MRIs obtained immediately post treatment. All cases showed a similar pattern observed as multiple dotted or linear parenchymal enhancements scattered in the frontal white matter of the targeted lesion and adjacent tissue, parallel to the direction of the gyrus. In some areas, we observed linear enhancement that exactly matched the dilated perivascular space, which was seen on high-resolution T2-weighted images. Contrast enhancement was observed in more number of patients after the second treatment than after the first. We targeted a mean volume of 21.1±2.7 cm3 in the bilateral frontal lobes. Immediate postprocedure Gd-enhanced MRI scans showed that 95.7%±9.4% of the target was well enhanced. MRI performed 3 months after the second procedure showed no enhancement in this area, indicating BBB closure. Additionally, predominantly in both frontal areas, meningeal enhancement was prominent in immediate posttreatment MRI and disappeared in follow-up MRI after 2 months. These findings might be due to contrast extravasation in the subarachnoid space and/or pial vessel engorgement.
T2*-weighted/GRE (SWAN) images showed low signal intensity dark spots
In SWAN images, low-signal intensity dots in the sonication area were observed in both patients with GBM and AD. T2*-weighted/GRE (SWAN) MRI of the targeted area exhibited dark signal spots in 64.1% of the GBM BBB opening targets. However, the low-signal intensity areas of the SWAN images did not always match the high-signal intensity T1 contrast enhancement areas. Spots were visible in either the T1 contrast images or SWAN sequences. When we combined the overlapped spot area of the T1 contrast and SWAN images, BBB opening was observed in 92.4% of the outlined area in the patients with GBM.
T1 and T2 (SWAN) showed the effects of MRgFUS were transient and reversible
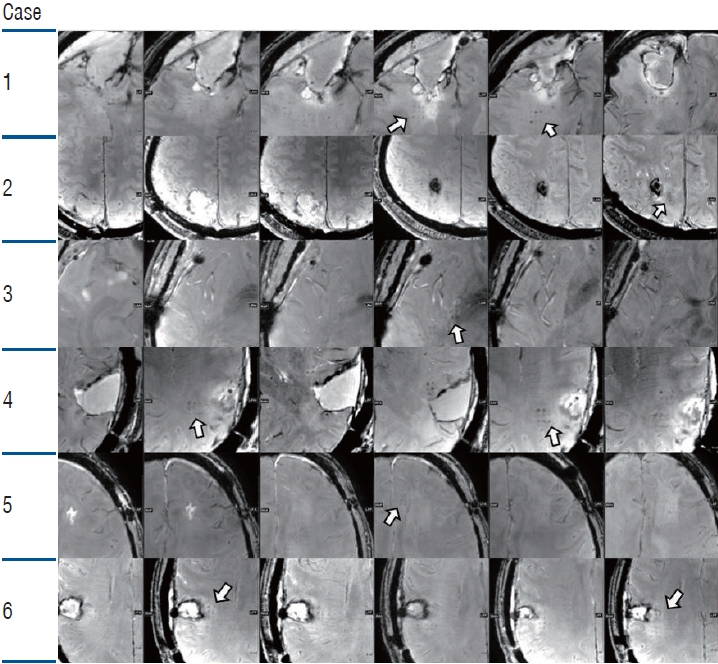
The T1 contrast-enhanced image showed high signal intensity in the sonication area, which reflects BBB opening due to extravasation of the contrast agents. In the follow-up image, the high signal intensity disappeared, and the iso-signal intensity returned, proving that BBB opening was transient (Fig. 3). On follow-up MRI after 2 months, all parenchymal enhancements disappeared, and no new lesions were detected. Dark spots were observed in the T2 SWAN images. These dark spots gradually attenuated after the follow-up images and some disappeared before the next BBB opening session; however, some spots remained in the high-energy sonication area (Figs. 4 and 5).

T1 contrast image for Alzheimer’s disease clinical study. Immediate post focused ultrasound magnetic resonance imaging (MRI) image (left row) and 1 month follow up MRI (right row) are shown. The T1 contrast image shows gadolinium enhancement in the blood-brain barrier opening area (left row). White arrow indicates marked contrast enhancement. The enhancement disappeared in the following image. The patient dropped out of the study in case 6.

Susceptibility-weighted angiography images of Alzheimer’s disease study. Immediate post focused ultrasound (FUS) magnetic resonance imaging (MRI) images (left row) show multiple dot shaped hypointense spots observed in the bilateral frontal lobes. Yellow circles indicate marked hypointense spots in the sonication area. The spot shape resembles the grid shape in the FUS system. Some spots attenuated and disappeared in the follow up MRI (right row). The patient dropped out in case 6.
Sequential susceptibility-weighted angiography (SWAN) images of glioblastoma study. Each row is the SWAN image immediately after the focused ultrasound (FUS) sonication. Magnetic resonance imaging images show multiple dot shaped hypointense spot observed in the sonication sites (white arrows). The spots resemble the grid shape in the FUS system. Note that the spot differs in location according to the sonication area, and in the follow-up image, most of the spots disappeared.
MRI showed no visible side effects of MRgFUS
None of the patients experienced any adverse events, such as intracerebral hemorrhage, edema, or newly enhancing lesions, in the sonicated areas during the six cycles of treatment. All patients with GBM had showed atleast a partial response to radiological intervention. No systemic or neurological worsening was observed. Furthermore, no brain edema, overt cerebral hemorrhage, or infarction was observed in the patients during or after the study. No significant parenchymal contrast enhancement was observed in non-targeted regions. T2 or diffusion signal remained unchanged in the targeted area, and edema or mass effect was absent. Gross parenchymal or extraparenchymal hemorrhage was absent.
DISCUSSION
Minimally invasive and reversible BBB opening using low-intensity FUS is an active area of research. FUS has been used in animal models of various diseases, including brain tumors [3,22,28,44], Parkinson’s disease [19], and AD [42]. Research has focused on enhancing the delivery of numerous therapeutic agents, such as chemotherapeutic agents, various drugs, antibodies, stem cells and viruses [5,13,33]. In mouse models of AD, ultrasound was used to disrupt BBB for delivering antibodies against beta-amyloid and tau protein or for removing plaques [17,32,42]. Most studies have been preclinical animal studies, and there are only a few clinical results obtained by sonication. A phase 1 clinical trial by Lipsman et al. [21] targeted the right frontal lobe and a phase 2 clinical trial by Rezai et al. [39] targeted the hippocampus and entorhinal cortex in patients with AD. Despite successful BBB opening, the clinical effect was not significantly beneficial in the two studies, which may be due to the limited area targeted by BBB opening.
FUS has the potential to open the BBB temporarily and reversibly, with minimal invasiveness. In our previous study of essential tremors, we occasionally observed transient BBB opening around the margin of the thalamotomy area.
Since fine modulation of BBB opening might enhance drug delivery to the brain, it is important to estimate the extent of local contrast agent leakage on MRI and to investigate whether FUS can achieve temporary BBB opening without significant side effects. In this study, we evaluated MRI findings after FUS-induced BBB opening performed in both patients with GBM and AD, with a wider extent and larger sonif ication volume compared with that in previous investigations. Reproducible BBB opening was observed in the sonication area of every patient treated with FUS with favorable clinical outcomes. Thus, MRgFUS holds promise for future studies focused on improving BBB mediated drug delivery. With this technique, we can explore drugs that were effective in vitro but were less effective in vivo or has not been used because of the inefficiency due to the BBB. Even drugs that were approved in the use of brain diseases for example, temozolomide, could improve efficacy. Therapeutic agents such as liposomal doxorubicin, 1,3-bis(2-chloroethyl)-1-nitrosourea, paclitaxel are being studied. Antibodies such as aducanumab for treating AD could be studied in increasing its efficacy. Currently we are ongoing new clinical studies for FUS-induced BBB opening including our phase 2 clinical study for AD patients and enhancing chemotherapy drug delivery in GBM patients.
Furthermore, post-treatment MRI immediately after MRgF US revealed transient leptomeningeal enhancement in the sub arachnoid space as well as parenchymal enhancement in the targeted area, which was concordant with the findings describ ed in the glymphatic system [30]. We also observed a linear bran ching enhancement pattern in the area that exactly matched the dilated perivascular space, indicating extravasated contra st material filled the dilated perivascular space as well as the subarachnoid space by BBB opening with FUS.
However, to safely conduct BBB opening, FUS parameters must be appropriately controlled to minimize hemorrhage and massive extravasation [41]. Although microbubbles enhance FUS, the dose, size, and type of microbubbles can significantly impact BBB permeability [29]. Therefore, substantial efforts have been made to identify the optimal FUS parameters for BBB opening. However, there is still heterogeneity in the parameters recommended by different research groups [27,41]. In an animal preclinical study, we determined safe and efficient parameters for BBB opening in rats [41]. However, these parameters may differ in humans.
In our clinical studies, we opened the BBB in a small area of 1 cm3 for several targets in our initial GBMTMZ group. Owing to the success and satisfactory results of BBB opening in patients with GBM, a larger area was targeted in the AD groups, including majority of the bilateral frontal lobes. Currently we are able to target larger areas for our new ongoing clinical trials (Fig. 6). Temporary T1 contrast enhancement was observed in all cases treated with BBB opening. In the SWAN image, spots with low signal intensity were observed in the BBB opening area. The low-signal spots disappeared or were attenuated in the follow-up SWAN imaging. However, several spots that received high energy appeared to remain at a low signal intensity.

A new ongoing blood-brain barrier (BBB) opening clinical study of drug delivery in recurred glioblastoma (GBM). A wider BBB opening area can be achieved than in the GBM temozolomide clinical study. A : Planning image with grid. B : Post focused ultrasound MR images, susceptibility-weighted angiography, T1, T1 contrast. Red circles in the (B) indicate the region of interest in the post operative images.
Attempts for larger BBB opening areas require a higher sonication energy, thus increasing the chance for more low-signal-intensity dots in SWAN images. However, most of the spots were reversible, and in these cases, the low signal intensity in the SWAN images disappeared and attenuated. However, no massive hemorrhage was observed on computed tomography or other MR sequences. These results are concordant with those of other research groups [21,23,39].
Normally, low-signal-intensity dark spots in the SWAN image refer to hemorrhage [12]. SWAN is a high-resolution 3D multi-echo gradient echo sequence that produces average weighting across images with different echo times to achieve higher susceptibility weighting [9]. Because of their sensitivity to susceptibility changes, SWAN sequences can depict intracranial paramagnetic blood degradation products, such as deoxyhemoglobin, methemoglobin, or hemosiderin [9].
Cerebral microbleeding (CMB) can be detected by SWAN imaging using small hypointense spots with a maximum size of up to 5 mm or even 10 mm [12]. Most cases are non-symptomatic; however, some studies have found a correlation with negative prognosis in certain diseases [45]. CMB is increasingly being detected because of the increased use of high-resolution MRI [12]. From a pathological perspective, microblee ding is a small accumulation of blood degradation products that can be detected using susceptibility weighted imaging [11]. CMBs are depicted with a true positive rate of 48–89% at 1.5 T or 3.0 T and T2*-weighted or susceptibility weighted imaging across a wide range of diseases [12].
In our study, hypointense spots were observed in the sonication area. Initially, we considered this as microbleeding, but as we observed dark spots, the pattern was slightly different from microbleeding. A few intense spots remained in the follow-up image, similar to cerebral microbleeding, but most of the other spots disappeared. There were no signs of hemorrhage in any of the other MR sequences. There were no abnormal neurological symptoms even though it was an eloquent area. The presumptive reason for the hypointense spot could be temporary blood pooling around the BBB opening lesion or minor red blood cell leakage. The linear branching enhancement pattern observed in the T1 contrast images exactly matched the dilated perivascular space, which could also be the cause of the low signal intensity in T2 SWAN images. In a BBB study of glioma by Anastasiadis et al. [2], histological examination revealed no significant differences or evidence of microhemorrages in FUS-treated tumor regions in four patients who received BBB opening. Regarding safety, these spot sizes and transience may indicate the limits for FUS treatment. Drug delivery efficiency or substrate permeability in patients with no spots and dark spots have to be investigated in future studies. Currently, the presence of such a spot is an important guideline for BBB opening at our institute during the FUS.
CONCLUSION
We demonstrated a safe BBB opening with FUS in clinical studies of patients with GBM and AD. Continuous efforts to increase the effectiveness of FUS for BBB opening may enhance CNS drug delivery strategies and the clearance of neurotoxic molecules, which has immense application in various pharmacological and neuroscience research fields.
Notes
Conflicts of interest
Hyun Ho Jung has been editorial board of JKNS since May 2017. He was not involved in the review process of this original article. No potential conflict of interest relevant to this article was reported.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Author contributions
Conceptualization : JWC; Data curation : KWC; Formal analysis : KWC; Methodology : KWC; Project administration : JWC; Visualization : KWC, SWH; Writing - original draft : KWC; Writing - review & editing : SWH, WSC, HHJ, JWC
Data sharing
None
Preprint
None
Acknowledgements
We would like to thank Itay Rachmilevitch, E.J. Kwon and our radiologists for their contribution.
