Fibrinolytic (Thrombolytic) Therapy for Post Intraventricular Hemorrhagic Hydrocephalus in Preterm Infants
Article information
Abstract
While the survival rate of preterm infants has increased dramatically over the last few decades, intraventricular hemorrhage and subsequent hydrocephalus remain major unsolved problems in neonatal intensive care. Once intraventricular hemorrhage occurs, severe neurological sequelae are inevitable. Treatment of this complicated pathology and achievement of favorable neurofunctional outcomes in fragile infants are crucial challenges for pediatric neurosurgeons. Fibrinolytic therapy, which chemically dissolves hematoma, is a promising and useful treatment method. In this paper, the historical background of fibrinolytic therapy for postintraventricular hemorrhagic hydrocephalus in preterm infants is reviewed and a recent method of fibrinolytic therapy using urokinase is introduced.
INTRODUCTION
Significant advances in neonatal intensive care have dramatically improved the survival rate of preterm infants, including those with extremely low birth weight (<1000 g at birth) [15,18,20,48,50]. Although the development of exogenous pulmonary surfactant replacement treatment and artificial respiratory therapy using high-frequency oscillation has brought about a breakthrough in overcoming respiratory distress syndrome in preterm infants, gastrointestinal perforation due to necrotizing enterocolitis and intraventricular hemorrhage (IVH) remain serious and life-threatening complications [34]. Fragile blood vessels in the germinal matrix layer are easily damaged, and IVH is known to occur in 15–36% of preterm infants [33]. In the 1970s, the severity of IVH was classified by Papile et al. [24], based on computed tomography scans; currently IVH patients are classified as IVH grades I–IV based on the ultrasonography findings described by Volpe [38,39]. IVH grades I and II are often self-limiting; however, higher-grade IVHs are frequently complicated by hydrocephalus (post intraventricular hydrocephalus, PIVHH) and are associated with an increased risk of neurodevelopmental disorders that cause not only cognitive impairment but also cerebral palsy [1,4,6,23]. Progressive ventricular dilatation results in white matter damage due to anatomical and physical factors, such as pressure and strain, that lead to ischemia. In addition, the adverse effects of free radicals and inflammatory damage after IVH should be recognized as a cause of white matter injury [10,30,31,35,47].
TREATMENT CONCEPT AND GOALS
For decades, the primary goal of PIVHH treatment has been to reduce mortality because the target patients are fragile premature infants. The next approach was to safely perform ventriculoperitoneal (VP) shunt surgery to treat the hydrocephalus and the subsequent challenge was to reduce the need for permanent VP shunt placement. The mission of current pediatric neurosurgeons is to reduce the risk of white matter damage and improve neurological outcomes [5,7,21,26,40].
How should pediatric neurosurgeons approach the treatment concept? The adverse effects of long-term neglect of highly enlarged ventricles and increased intracranial pressure on the infant brain, as well as those of the hematoma itself and various inflammatory cytokines associated with its dissolution products, should be considered [40]. The oversimplified idea that everything will be resolved if a VP shunt can somehow be placed, is misguided.
The following therapeutic concepts should be considered to improve neurodevelopmental outcomes : 1) continuous intracranial pressure control from the early PIVHH stage; 2) morphological recovery of the brain mantle volume as soon as possible; 3) rapid hematoma dissolution and wash out; 4) reduction of free radicals and inflammatory cytokines; 5) restoration of physiological cerebrospinal fluid (CSF) dynamics; and 6) avoidance of permanent VP shunt placement. These therapeutic approaches contribute to the minimization of periventricular white matter damage [5,26,40].
Based on the results of clinical studies to date, two treatments are thought to be promising [5], namely neuroendoscopic lavage (NEL) [3,32,36] and fibrinolytic therapy combined with ventricular drainage. This study reviews the history and challenges of fibrinolytic therapy for PIVHH.
THE DRIFT (DRAINAGE, IRRIGATION, AND FIBRINOLYTIC THERAPY) TRIAL
Whitelaw et al. [46], at the University of Bristol, United Kingdom, reported the first fibrinolytic treatment for PIVHH in preterm infants. He has reported many excellent clinical studies in this field over the years, and his work deserves special mention. Whitelaw et al. [41,42,45] carried out an epoch-making fibrinolytic clinical study called the DRIFT trial using tissue plasminogen activator (tPA), the results of which are renowned worldwide.
Their clinical research was based on meticulous accumulation of data. They first reported that tPA and fibrin degradation products were present in posthemorrhagic CSF, and then found that plasminogen concentrations were very low and plasminogen activator inhibitor-1 was present at high concentrations [13,43,44]. They subsequently validated these indicators of the coagulation and fibrinolytic systems, established the feasibility of fibrinolytic therapy for PIVHH, and conducted several preliminary clinical studies [46,47]. However, no encouraging results were obtained. The reason for this was thought to be that fibrinolytic therapy was started 2–4 weeks after the onset of IVH, when hydrocephalus had already developed irreversibly [45].
They later attempted a unique therapy combining fibrinolytic therapy with intraventricular injection of tPA and continuous irrigation soon after the onset of IVH in premature infants, which is the globally known DRIFT trial [41,42,45].
The phase 1 trial was conducted in 2003 [45]. In this phase of the trial, 24 preterm infants (median birth weight, 1150 g) were treated with tPA for ventricular dilatation after IVH. The aim of the treatment was to intervene at an early stage after IVH, before ventricular dilatation had progressed substantially, particularly before hydrocephalus had developed. Therefore, the median ventricular width was 16 mm and the median age at the initiation of treatment was 17 days. It can be said that this was significantly different from the conventional treatment concept in which treatment was initiated when severe ventricular dilatation had developed. In the past, conventional treatment was started when the fontanelle was bulging or bradycardia occurred.
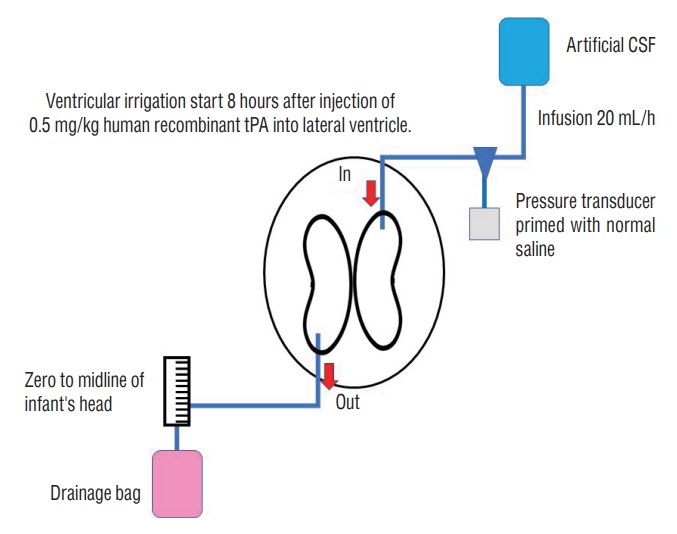
The specific method was as follows [41] (Fig. 1) : first, ventricular drainage surgery (and, if possible, ventricular reservoir placement) was performed. The patient was observed for 1–2 hours after surgery, until their general condition had stabilized. Thereafter, 0.5 mg/kg human recombinant tPA was injected into the ventricle and left for 8 hours. A single injection of tPA was administered. Ventricular lavage was subsequently performed using artificial CSF. The intracranial pressure was continuously monitored and maintained at <7 mmHg. Irrigation was performed for 72 hours but could be continued for up to a week if the CSF had not been cleansed from the appearance of cola to that of white wine. A VP shunt was inserted when there was a continuous need for CSF tapping or when there was persistent accelerated head enlargement.
The results of these clinical studies are remarkable. The efficacy of the treatment far exceeded that of standard treatments at that time and the results were considered superior to those obtained using existing standard treatment. Seventeen of the 23 survivors (74%) did not require permanent VP shunt placement. Two infants developed reservoir-associated infections and two had a second IVH. Of the 19 survivors ≥12 months post-term, eight were normal, seven (37%) had a single disorder, and four (21%) had multiple disorders. Shunt surgery was dramatically reduced compared with historical controls with similar treatment criteria, and single and multiple disability rates were also reduced. The DRIFT trial could reduce periventricular brain damage and avoid the development of hydrocephalus by maintaining a sustained low intracranial pressure and reducing free iron and inflammatory and profibrotic cytokines during the treatment of fragile preterm infantile IVH.
A multicenter randomized controlled trial (RCT) was designed to objectively evaluate the efficacy and safety of DRIFT [42]. The comparative standard treatment group underwent repeated CSF removal (10–20 mL/kg) by lumbar puncture, followed by placement of a ventricular reservoir to remove the same volume of CSF. Seventy infants were recruited; 34 preterm infants were randomized to the DRIFT cohort and 36 to the standard treatment cohort. However, the results were disappointing. Of the 34 infants who were assigned to DRIFT, two died and 13 underwent shunt surgery (dead or shunt, 44%). Of the 36 infants assigned to standard therapy, five died (one with a shunt) and 13 survived shunt operations (dead or shunt, 50%). This difference was not statistically significant (p=0.80). Twelve (35%) of the 34 infants who received DRIFT had secondary IVH, compared with three (8%) of 36 infants in the standard treatment group; this difference was statistically significant (p=0.014). Secondary IVH was therefore associated with an increased risk of subsequent shunt surgeries.
The results presented by this RCT were highly significant and created great doubt in the efficacy and safety of DRIFT therapy, which subsequently discouraged the introduction of fibrinolytic therapy to treat IVH in preterm infants. The guidelines even stated that fibrinolytic therapy was contraindicated [22]. Reduction of the occurrence of secondary IVH has emerged as an important issue in fibrinolytic therapy.
Luyt et al. [21] and Whitelaw et al. [41,42,45] continued to publish important results focusing mainly on neurodevelopmental outcomes. They randomly assigned 77 preterm infants with posthemorrhagic ventricular dilatation to either DRIFT or standard treatment and assessed severe disability, including severe sensorimotor disability and cognitive disability, at 2 years of corrected age [42]. The assessment of treatment efficacy was based not only on the necessity of VP shunt placement, but also on functional prognostic aspects. Of the 39 infants assigned to DRIFT, 21 (54%) died or were severely disabled versus 27 of 38 (71%) in the standard group. Among the survivors, 11 of 35 (31%) in the DRIFT group had severe cognitive disability compared to 19 of 32 (59%) in the standard group. The median mental development index was 68 and <50 in the DRIFT and standard care groups, respectively. Although a previous RCT showed that DRIFT treatment failed to decrease the need for a VP shunt and had a high incidence of secondary IVH, DRIFT reduced severe cognitive disability in survivors and overall death or severe disability. Furthermore, they recently published a report on the prognosis of neurological function after 10 years [21]. Of the 77 randomized children, 12 died, two could not be traced, 10 did not respond, and one declined at the 10-year follow-up. In total, 28 in the DRIFT and 24 in the standard treatment group were assessed by examiners who were blinded to the intervention. The mean cognitive quotient scores were 69.3 and 53.7 in the DRIFT and standard treatment groups, respectively. The survival rate without severe cognitive disability was 66% in the DRIFT and 35% in the standard treatment group.
The DRIFT trial, designed to maintain low intracranial pressure, alleviate brain distortion, and reduce free iron and cytokines caused by IVH, was the first intervention for PIVHH in preterm infants to objectively demonstrate sustained cognitive improvement.
However, the fact that intraventricular administration of tPA could cause a high rate of secondary IVH was undeniable [41], and a new therapeutic strategy would be required to demonstrate the safety of fibrinolytic therapy.
CONVERSION FROM tPA TO UROKINASE (UK)
The efficacy of fibrinolysis by intraventricular infusion of UK for IVH in adult cases was first reported in the 1990s [2,28,37]. Secondary bleeding has rarely been reported as a serious complication. Furthermore, in an animal experimental study comparing the effects of tPA and UK in fibrinolytic therapy, only UK significantly improved neurological prognosis, which could be explained by the fact that UK, in contrast to tPA, fails to promote inflammatory processes and neurotoxicity [12].
However, there are few reports of fibrinolytic therapy using UK in the treatment of preterm IVH. Hudgins et al. [17] reported that shunt requirements were reduced only in the low-dose group, and they also observed a decrease in the requirement for revisions of shunt malfunctions. According to a report by Hansen et al. [14], all infants required shunt surgery, but fibrinolysis using UK was a safe treatment without serious complications.
Although tPA has a stronger pharmacological effect than UK, the possibility of treatment using UK should be thoroughly investigated based on previous reports.
Recently, a unique treatment strategy that combines continuous ventricular drainage management with repeated intraventricular infusions of UK for predominantly grade IV IVH in extremely low birth weight infants, was reported by Park et al. [27]. The priority was to avoid secondary bleeding, and repeated injection of a relatively low dose of UK (6000 IU) into the ventricle was a major difference from DRIFT treatment. As indicated above, the DRIFT trial used the method in which tPA is administered only once. The frequency of UK injections was initially limited to four times a day for a maximum of 5 days. After the safety of fibrinolytic therapy using UK was confirmed in serial cases, the frequency increased to eight times per day, and the treatment was continued for up to 14 days in severe cases (Fig. 2).
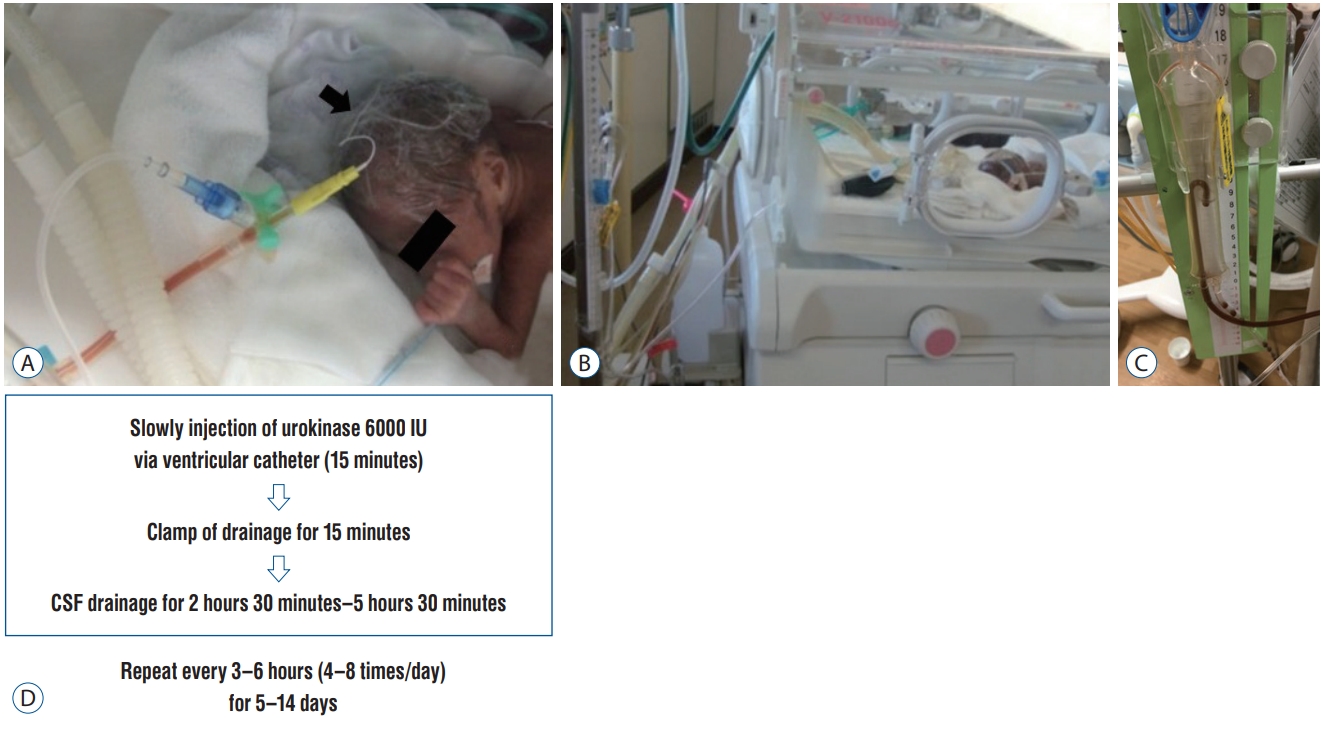
Fibrinolytic (thrombolytic) treatment. A : A very thin catheter is used for external ventricular drainage (indicated by the arrow). A urokinase (UK) infusion syringe pump was connected to a three-way cock. B and C : Dissolution of the hematoma by UK injection and drainage of bloody cerebrospinal fluid (CSF). D : Protocol of UK intraventricular injection. CSF : cerebrospinal fluid.
Drainage volumes were checked hourly. The pressure setting was adjusted frequently, and the daily volume of bloody CSF drainage was approximately 10–15 mL (Fig. 2B and C). During therapy, transfontanelle ultrasonography was performed twice daily to assess the occurrence of secondary hemorrhaging and to monitor ventricle size.
Serial ultrasonography confirmed that UK ensured early lysis of the hematoma. Fibrinolytic therapy effected rapid changes in the drained CSF (Fig. 3).
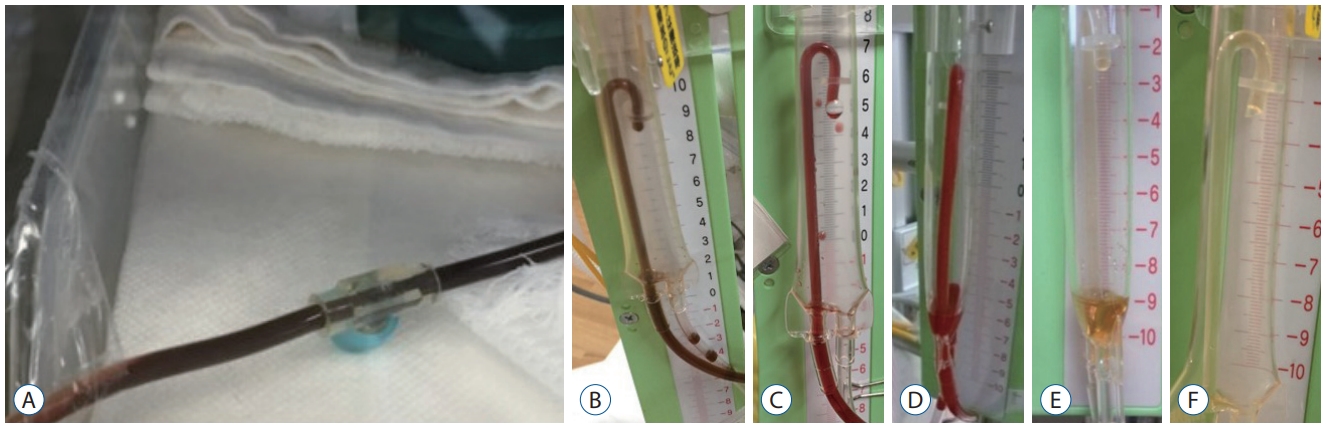
Changes in bloody cerebrospinal fluid resulting from fibrinolytic therapy. A : Before starting treatment. B : Day 1 of treatment. C : Day 2. D : Day 4. E : Day 6. F : Day 10.
No troublesome complications occurred during UK-based fibrinolytic therapy.
First, no complications occurred during ventricular drainage surgery. This was because a very thin catheter originally designed for intravenous access was used, and percutaneous ventricular puncture was always performed under ultrasonographic guidance (Fig. 4). With this method, safe surgery was possible even if the diameter of the anterior horn of the lateral ventricle was 4 mm.

A : A 24-gauge catheter (0.63 mm in diameter, PI catheter kit, ArgyleTM; Cardinal Health, Tokyo, Japan). B : Percutaneous ventricular puncture with a venous access needle. C : Insertion of a very thin catheter into the lateral ventricle.
Secondary hemorrhage associated with fibrinolytic therapy and CSF infection during external ventricular drainage (EVD) management did not occur.
It was necessary to secure the subcutaneous tunnel as far as possible (at least 4 cm or more) to avoid drainage infection resulting from leakage of CSF from the wound outlet (Fig. 5A). Catheter occlusion can occur with the use of very thin catheters. Leaving an occluded catheter could cause a drainage infection, and removal and replacement were performed without hesitation. To prevent CSF leakage, the wound was minimized and tightly covered with surgical film dressings (Fig. 5B).
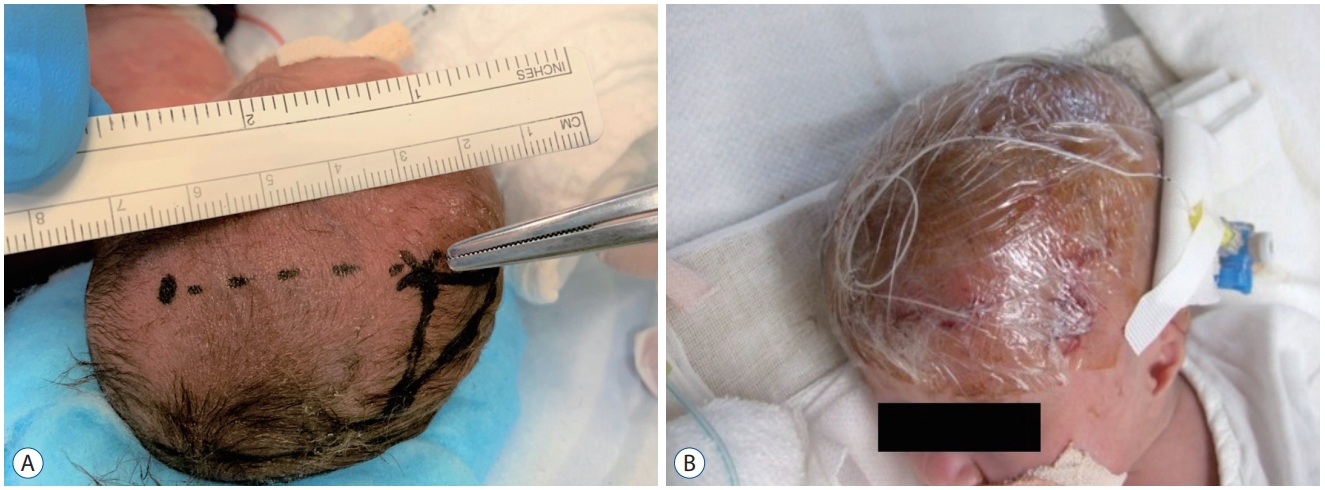
A : Subcutaneous tunnel of 4 cm or more and minimal surgical wounds. B : Surgical wounds are tightly covered with surgical film dressings.
Notably, no secondary hemorrhage due to fibrinolysis was observed in this study. This result differs greatly from those described in the DRIFT trial. The use of UK rather than tPA was the most important factor, and the dosing regimen of intermittent and repeated injections of low-dose fibrinolytics several times per day was also related.
Despite all 21 patients with IVH grade IV receiving fibrinolytic therapy, 86% were able to avoid VP shunt placement. Furthermore, when assessing useful motor function of the upper and lower extremities and language function at a corrected age of 36 months, the fibrinolytic therapy group using UK achieved significantly better functional outcomes (good, 76%; poor, 24%) than the standard treatment group (good, 40%; poor, 60%).
ILLUSTRATIVE CASES (FIBRINOLYTIC THERAPY USING UK)
Case 1
A male infant was born at 25 weeks and 5 days of gestation and weighed 818 g. Grade IV IVH developed on the morning of day 2 after birth (Fig. 6A). His respiratory and circulation was unstable, and the hematoma increased in size. A large hematoma was confirmed in the evening of the same day (Fig. 6B). Since this infant was unlikely to survive without early active intervention, EVD surgery was performed, and fibrinolytic therapy was initiated on the day of bleeding. UK injections were administered every 3 hours (eight times a day). Daily ultrasonography revealed effective and rapid dissolution of IVH and intracerebral hemorrhage (Fig. 6C and D). Fibrinolytic therapy was completed in 7 days, and management of EVD in 28 days. No recurrence of the ventricular dilatation was observed. Injury around the lateral ventricle was mild and minimal (Fig. 6E). PIVHH was cured without the requirement of a permanent VP shunt.

A : Ultrasonography on day 2 showing bilateral intraventricular hemorrhage (IVH) mainly in the right lateral ventricle (yellow arrows). B : A massive hematoma is confirmed after several hours post-onset (red arrows). Day 8 (C), Day 12 (D) : IVH and intracerebral hemorrhage dissolve effectively and rapidly. Day 200 (E) : T2-weighted magnetic resonance imaging revealing only mild white matter damage around the right lateral ventricle (blue arrows) without requiring permanent ventriculoperitoneal shunt placement.
Case 2
A male infant was born at 25 weeks and 1 day of gestation and weighed 663 g. Grade IV IVH occurred on the third day of life. The IVHs were severe, and the lateral ventricles increased in size daily (Fig. 7A). EVD surgery was performed on postnatal day 7 and fibrinolytic treatment was initiated. The hematomas were effectively lysed and ventricular enlargement rapidly improved (Fig. 7B and C). Fibrinolytic therapy was completed in 14 days, and EVD management on the same day. Magnetic resonance imaging showed mild periventricular white matter injuries (Fig. 7D). Treatment of PIVHH without permanent VP shunts was highly favorable.

A : Ultrasonography on day 4 showing bilateral intraventricular hemorrhage. The hematomas are very large (red arrows). Day 10 (B), Day 15 (C) : hematomas are significantly dissolved, and ventricular dilatation improves rapidly. Day 195 (D) : T2-weighted imaging revealed minimal white matter damage around the right lateral ventricle (blue arrows) without requiring permanent ventriculoperitoneal shunt placement.
THE IDEAL TREATMENT STRATEGY FOR PIVHH IN PRETERM INFANTS
NEL and fibrinolytic therapy may be two aggressive and promising treatments for PIVHH in preterm infants. Thirteen clinical studies have been conducted since 2000 [3,8,9,11,16,27,29,32,36,41,42,45,49]. Clinical outcomes, including VP shunt requirement rates, complication rates such as infection and secondary bleeding, and cognitive outcomes, were similar between the two treatment groups [19,26] (Table 1). However, advanced surgical techniques are essential for performing NEL, and its application to extremely small heads and slightly enlarged ventricles is limited.
Considering various factors, the ideal treatment strategies for PIVHH in extremely low birth weight infants are now considered to be the following [5,25,26] (Fig. 8) : step 1, EVD management combined with fibrinolytic therapy using UK (not tPA); step 2, NEL, if possible; and step 3, Either intermittent CSF evacuation using a ventricular reservoir, or a ventriculosubgaleal shunt, which has long been considered the standard treatment. When progressive PIVHH cannot be controlled using other treatment methods, a VP shunt is the final choice. However, it should be noted that the incidence of shunt-related complications is extremely high, and multiple additional surgical treatments may be required.
CONCLUSION
The therapeutic goal for PIVHH in preterm infants is not only to focus on how to successfully perform VP shunt surgery but also to improve neurofunctional outcomes. It would be ideal if VP shunt placement could be avoided; fibrinolytic therapy is being recognized as an effective treatment option. The use of UK as a fibrinolytic agent is preferred because there is a high risk of secondary bleeding with the use of tPA. As NEL is only applicable in a limited number of cases, fibrinolytic therapy might be given priority in extremely small infants.
Multicenter comparative research is required to evaluate which treatment method, including fibrinolytic therapy, is the safest and most effective.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Author contributions
Conceptualization : YSP; Data curation : YSP; Formal analysis : YSP; Methodology : YSP; Project administration : YSP; Writing - original draft : YSP; Writing - review & editing : YSP
Data sharing
None
Preprint
None
Acknowledgements
I would like to express my sincere gratitude to Andrew Whitelaw (Professor Emeritus, Bristol University) for his valuable advice, and to convey my deepest respect for his outstanding and excellent work over the years.