Craniosynostosis in Growing Children : Pathophysiological Changes and Neurosurgical Problems
Article information
Abstract
Craniosynostosis is defined as the premature fusion of one or more cranial sutures resulting in skull deformity. Characteristically, this disorder can cause diverse neurosurgical problems, as well as abnormal skull shape. Intracranial hypertension, hydrocephalus, Chiari malformation and neuropsychological dysfunction are the major neurosurgical concerns in children with craniosynostosis. In this review article, we investigate pathophysiology, characteristics and proper neurosurgical management of these neurosurgical issues, respectively.
INTRODUCTION
By definition, craniosynostosis is characterized as the premature fusion of one or multiple cranial sutures and consequent abnormal skull shape. However, this disorder is not only confined to skull deformity, but can cause various neurosurgical issues in growing children. Intracranial hypertension, hydrocephalus and Chiari malformation are the representative problems that can be combined with craniosynostosis. Moreover, these neurosurgical problems, as well as skull deformity, have a risk of brain insult and can be associated with neurologic and cognitive dysfunction. In this article, we reviewed the literature regarding pathophysiology, characteristics and proper neurosurgical management of the neurosurgical concerns that can accompany craniosynostosis.
SKULL GROWTH AND INTRACRANIAL VOLUME IN CRANIOSYNOSTOSIS
Understanding skull growth is essential for explaining other pathologic conditions in craniosynostosis. Over recent decades, there is a noticeable change in concept on the relationship between craniosynostosis and intracranial volume (ICV). The long standing concept was that premature fusion of cranial sutures caused by craniosynostosis would restrict growth of the brain by limiting the amount of space within the cranial vault11520). Therefore, it was widely accepted that most cases of cranaiosynostosis would result in reduced ICV.
However, this concept is changed with the development of advanced image-manipulation techniques. Many studies using accurate measurement of ICV by either computerized tomography or magnetic resonance images conclude that ICV in majority of children with craniosynostosis is within normal limits and even that children with Apert syndrome tend to have larger ICV than normal2426424351). Other studies demonstrate that patients with craniosynostosis are born with a significantly smaller ICV, but at around the age of 6 months they achieve normal volume51). According to these studies, in craniosynostosis, the skull struggles to maintain normal ICV during growth, despite the obstacles caused by fused sutures, probably due to compensatory growth through unaffected suture sites. Only patients with the rare forms of pansynostosis may have a true restriction of skull growth, resulting in microcephaly and a small ICV51). However, exception is still found in Apert syndrome, which is characterized by greater than normal ICV despite multiple suture involvement26).
INTRACRANIAL PRESSURE IN CRANIOSYNOSTOSIS
Intracranial hypertension or increased intracranial pressure (ICP) is extensively observed in children with craniosynostosis31618345358). The risk of increased ICP is dependent on the number of involved sutures. Whereas about half of the patients with multisuture involvement show increased ICP18), the incidence of cases in patients with single suture craniosynostosis ranges from around 15% to 20%1747565961). Furthermore, increased ICP is more frequently observed in syndromic craniosynostosis patients and the prevalence depends greatly on the type of syndrome (Table 1)47575859).
Originally, the decreased ICV associated with craniosynostosis was thought to account for the increased ICP. However, this mechanism is likely only one of several factors contributing to increase ICP, even though decreased ICV may be an important contributing factor in a few cases2). Most patients with craniosynostosis have normal ICV, however, increased ICP can exist in the presence of normal ICV. Indeed, several factors are known to contribute to a pathological increase in ICP in patients with craniosynostosis. According to previous studies, abnormal cerebrospinal fluid (CSF) circulation, hydrocephalus, upper airway obstruction, and intracranial venous congestion consequent to impaired venous drainage contribute to raise ICP5661). Among these, hindrance of CSF absorption in craniosynostosis is known to be the major cause of increased ICP. This is supported by the clinical experience of more common increased ICP on the involvement of a midline suture such as sagittal or metopic, as compared to than a single coronal suture5961). Moreover, abnormal dilatations of the subarachnoid space are commonly found in children with craniosynostosis, and may imply some disturbance in CSF absorption59).
Various studies propose mechanisms of impaired CSF absorption in cases of craniosysnostosis. One study suggests that direct compression of the superior sagittal sinus in an abnormally narrow bone groove, created by the premature fusion of the sagittal suture might account for CSF malabsorption56). Moreover, according to another report, the bony changes along the sagittal suture may directly impair the absorption of arachnoid granulations in sagittal synostosis29). Highly transmitted brain pulsations are a different suggested mechanism for the dilation of subarachnoid spaces underlying the prematurely fused sutures in infants with coronal or lambdoid synostosis3).
In addition to impaired CSF absorption, upper airway obstruction is a noticeable contributor to increased ICP. It is proposed that carbon dioxide retention during obstructive episodes and cerebral flow changes during sleep could raise ICP5860). Therefore, prolonged ICP monitoring including sleep ICP may be important to differentiate airway problem in craniosynostosis patients from other etiologies. To manage raised ICP in this situation, nocturnal positive airway pressure or maxillofacial advancement procedures are recommended rather than craniofacial reconstruction23).
Increased ICP can cause many clinical problems for craniosynostosis patients. Chronically raised ICP in patients with craniosynostosis can lead to optic atrophy and blindness, and there is strong evidence to suggest that it may impair intelligence. However, most patients with craniosynostosis show a slowly progressive clinical course. Hence, the majority of patients have no warning signs or symptoms in the incipient stage, and the diagnosis may be possible only at an advanced stage of intracranial hypertension175862). Therefore, clinical suspicion is very important.
Similar to usual cases with of elevated ICP, the most common symptoms of craniosynostosis patient with increased ICP are also headache, vomiting, irritability, bulging fontanel, and altered mentality2). However, most symptoms are extremely non-specific for the craniosynostosis patients. A study on 121craniosynostosis patients using intraparenchymal wires shows poor correlation of symptoms with increased ICP; headache, irritability, and nausea in only 19%, 25%, and 12%, respectively, of all patients17).
On the contrary, fundus oculi may better reflect the ICP and papilledema closely correlates with increased ICP62). Therefore, ophthalmologic examination for the presence of papilledema is very effective as a screening tool for elevated ICP in craniosynostotic patients. However, such ocular finding occurs late in the clinical course of increased ICP, and is nearly followed by irreversible ocular damage with optic atrophy. Recently, visual evoked potentials are emphasized for the early detection of raised ICP. A previous report describes continued deterioration in visual evoked potentials as the only sign of elevated ICP in a patient with no change in visual acuity and optic disc appearance34).
To properly manage the raised ICP, understanding of exact etiology of intracranial hypertension should be required. Without adequate evaluation of pathophysiology, cranial vault expansion shows limited ability to compensate intracranial hypertension, as long as there is persistence of other causative factors of the raised ICP, such as hydrocephalus, impairment of CSF circulation or venous sinus circulation and upper airway obstruction56).
UPPER AIRWAY OBSTRUCTION IN CRANIOSYNOSTOSIS
In formulating a lifelong strategy to prevent neurocognitive loss and maximize development for any child with syndromic craniosynostosis, avoiding prolonged period of hypoxia is an important focus. In early infancy, central sleep apnea (typically associated with an acquired Chiari malformation, which results in brainstem compression) is less commonly identified as the cause for impaired ventilation, and obstructive sleep apnea (OSA) is far more likely. OSA may be multifactorial, but is most often directly related to mid facial hypoplasia. This hypoplasia elevates the palate, which correspondingly reduces the size of the nasal airway.
Use of continuous positive airway pressure masks and tonsillectomies are more conservative treatments for cases diagnosed with OSA. On the other hand, temporary tracheostomies lower mortality rates for the more severe patterns of syndromic craniosynostosis, and should be considered in all infants and younger children who are non-responsive to more conservative therapies19). Typically, the incidence for airway compromise can be expected to increase with age, as the mid facial hypoplasia becomes more progressive. Occasionally, Le Fort III distraction osteogenesis (DO) is indicated in syndromic craniofacial patients with midface hypoplasia involving the nasal, maxillary and zygomatic complex, shallow orbits, exophthalmos, upper airway obstruction and obstructive sleep apnea, class III malocclusion and an overall severe facial aesthetic imbalance. Advancement of the midface with Le Fort III expands the nasopharynxand oropharynx, often allowing for tracheostomy decannulation. In addition, by advancing the midface using Le Fort III distraction osteogenesis, the abnormal proptotic position of the globe relative to the orbital rim are corrected, thus preventing amblyopia, corneal exposure with subsequent exposure keratitis, keratoconjunctivitis sicca and infection leading to corneal ulceration, cataracts, and possibly vision loss41).
HYDROCEPHALUS IN CRANIOSYNOSTOSIS
Progressive hydrocephalus in craniosynostosis should be distinguished from nonprogressive ventriculomegaly. Ventricular dilatation in craniosynostosis may represent either shunt-dependent progressive hydrocephalus or shunt-independent ventriculomegaly61040). Progressive hydrocephalus is mainly a clinical issue in craniosynostosis.
The association between hydrocephalus and craniosynostosis is well documented. The incidence of hydrocephalus varies to a great extent according to the number or types of affected suture and whether or not craniosynsostosis is syndromic. A previous study reports that only 0.28% patients (4 of the 1447 cases) of nonsyndromic craniosynostosis present with progressive ventricular dilation that require shunt insertion, whereas 12.1% patients (34 of the 280 cases) of syndromic craniosynostosis require surgical treatment for progressive ventricular dilation6).According to another study, of the 315 cases of nonsyndromic craniosynostosis, 32 patients (10.2%) show ventricular dilatation and shunt operation is required in only 8 patients (2.5%)10). On the other hand, syndromic craniosynostosis shows a higher incidence, with 81 patients (44.0%) among the 315 cases of syndromic craniosynostosis, showing ventricular dilatation and 20 patients (6.3%) as shunt-dependent in the same study10). Moreover, a tremendous difference in incidence between complex craniofacial syndromes is consistently reported in many studies (Table 2)610283638404446).
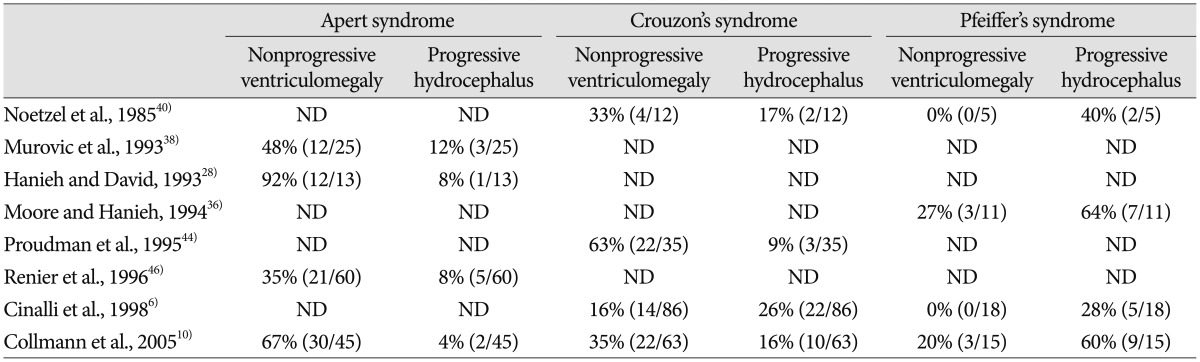
Reported prevalence of nonprogressive ventriculomegaly and progressive hydrocephalus in syndromic craniosynostosis
Hydrocephalus in craniosynostosis can occur as the result of either CSF flow obstruction or impaired CSF absorption, since both are related to the deformity of the skull or to coincidental disorders independent from craniosynostosis10). In light of the finding that the incidence of hydrocephalus in nonsyndromic craniosynostosis is equivalent to that of the general population unaffected by craniosynostosis, the few cases of progressive hydrocephalus in isolated nonsyndromic craniosynostosis may almost always be attributed to coincidental disorders independent from craniosynostosis, such as ventricular hemorrhage, meningitis, aqueductal stenosis, neural tube defects and other congenital disorders61011212540).
On the contrary, the opinion that progressive hydrocephalus in syndromic craniosynostosis is a coincidental disorder is now largely abandoned10). Even though the causative mechanism of progressive hydrocephalus in syndromic craniosynostosis is a controversial issue, there is some consensus on pathogenic factors. One hypothesis involves mechanically increased CSF outflow resistance due to constriction of the posterior fossa4510112260) and the other, CSF malabsorption resulting from venous outflow obstruction6102248). The former hypothesis is supported by the finding that most cases of progressive hydrocephalus in syndromic craniosynostosis with few exceptions show crowded CSF spaces of the posterior fossa, a small fourth ventricle, and a Chiari-like anomaly4562260). However, this theory also has a weak point, since posterior fossa decompression often fails to sufficiently restore normal CSF circulation and improve hydrocephalus410). The latter hypothesis of impaired CSF absorption due to insufficient venous outflow involves the concept of venous sinus hypertension as a major factor in progressive hydrocephalus2648). The venous sinus hypertension can be caused by a stenosis of the jugular foramen which was documented in syndromic cranisosynostosis. For example, one study identifies fixed venous sinus hypertension caused by a stenosis of the jugular foramen in some hydrocephalic Crouzon's patients. Moreover, this study also shows normalization of CSF pressure and ventricular size post-venous bypass between the transverse sinus and the jugular vein in one of the patients48). Another study demonstrates that there is not only jugular foramen stenosis but also an extensive venous collateral network in syndromic craniosynostosis6). Since most patients with progressive hydrocephalus simultaneously show clinical finding of both venous outflow obstruction and compromised posterior fossa, a combined action of both mechanisms is now advocated. One promising hypothesis is that venous hypertension causes the impaired CSF absorption as well as brain swelling resulting in crowded posterior fossa22); or that venous hypertension aggravates the pre-existent cephalo-cranial disproportion by venous engorgement660).
In general, the diagnosis of hydrocephalus is uncomplicated because rapidly progressive ventricular dilatation prior to any surgical intervention is identified in about 30–50% of the patients affected by hydrostatic hydrocephalus610). However, the diagnosis of progressive hydrocephalus in craniosynostosis may be less easily established because outer force of ventricular distention is limited by the inner force of restriction resulting from the rigid synostotic skull. Therefore, in a large proportion of shunt-dependent patients, the hydrocephalic condition may be latent. Actually, ventricular enlargement occurs after cranial remodeling in about half of shunt-dependent patients10). In these cases, the indication for shunting is mainly based on severity of ventricular dilatation or evidence of persistently raised ICP, which is confirmed by papilledema or abnormality on visual evoked potentials or by direct ICP monitoring10).
Occasionally, clinicians are faced with a challenging decision on the order of treatment for cases of somewhat hydrostatic hydrocephalus with increased ICP with craniosynostosis. In this clinical situation, unless there are reliable diagnostic criteria of progressive hydrocephalus, it is generally recommended to first operate on the craniosynostosis and subsequently to carefully survey the aggravation of ventricular size and ICP610). This suggestion is based on the clinical experience that some of patients with mild or even moderate progressive ventricular enlargement actually will remain in a shunt-independent state after adequate cranial expansion1127). Long-term surveillance is essential in this situation.
CHIARI MALFORMATION IN CRANIOSYNOSTOSIS
Chiari malformation is characterized by a downward herniation of the caudal part of the cerebellum and/or medulla oblongata through the foramen magnum. The association between Chiari malformation and craniosynostosis is well described49). A promising hypothesis on pathogenesis of Chiari malformation involves overcrowding in the posterior cranial fossa due to a normal-sized hindbrain in the underdeveloped occipital bone that secondarily induces a downward herniation of the brain16); thus, craniosynostosis can frequently accompany Chiari malformation39). In fact, Chiari malformation occurs in patients with both syndromic and nonsyndromic craniosynostosis. However, the patients with syndromic craniosynostosis are more frequently associated with Chiari malformation, because it affects more sutures and consequently has high possibility to involve lambdoid suture. According to previous literature, the incidence of Chiari malformation is as high as 70% in Crouzon's syndrome5737), 50% in Pfeiffer's syndrome and 100% in Kleeblattschädel deformity4). Interestingly, Chiari malformation is not common in Apert syndrome. One study reports that Chiari malformation is found in only 1.9% of patients with Apert syndrome5). Another study explains that the cranium synostosis occurs very early for the coronal suture (median 5 months) and later for the sagittal and lambdoid sutures (51 and 60 months, respectively) in the Apert syndrome, whereas in Crouzon's syndrome the sagittal and lambdoid sutures close very early (median 6 and 21 months, respectively)7).
Incidence of Chiari malformation is greatly influenced by involved suture type and number.
One study demonstrates that patients with single-suture lambdoid synostosis (55.6%) or multisuture craniosynostosis including lambdoid suture (57.1%) are much more likely to have associated Chiari malformation than all other patients with craniosynostosis (0–10.5%)55). Even though nonsyndromic single suture craniosynostosis affecting other than lambdoid sutures can also accompany Chiari malformation, their incidence is reportedly very low at up to 5.6%33).
While the synostosis of the lambdoid suture is a very important factor for the pathogenesis of Chiari malformation, other factors also affect the development of Chiari malformation. Hydrocephalus is a representative medical condition that is related with Chiari malformation. In syndromic craniosynostosis, Chiari malformation is observed in the majority (88%) of children affected by hydrocephalus6). Thus the possible pathogenesis of Chiari malformation in craniosynostosis includes the interactions such as the crowding of the posterior fossa resulting from the synostosis of the lambdoid suture or others, the jugular foramen stenosis with venous sinus hypertension and the increased CSF outflow resistance7).
According to previous literature, approximately one-third of patients who have Chiari malformation associated with craniofacial disorders are either symptomatic or have a syringomyelia7). Hence, most authors advocate screening of patients with syndromic craniosynostosis and patients with lambdoid synostosis with brain and spine magnetic resonance imaging prior to surgical correction of the craniosynostosis55).
Like any other Chiari malformation without craniosynostosis, symptoms of Chiari malformation in craniosynostosis can vary depending on severity of herniation and can even be life-threatening such as apnea, stridor due to vocal cord palsy and progressive brain stem dysfunction, especially in very young children.
While there is widespread agreement to treat patients with symptoms, the treatment of choice for Chiari malformation in craniosynostosis remains controversial. In some cases, Chiari decompression can be achieved simply at the time of a planned craniosynostosis repair55). Furthermore, one report showed that a supratentorial cranial expansion resulted in resolution of an acquired Chiari malformation14). However, several groups recommend posterior fossa expansion surgery as the treatment of choice for all cases of Chiari malformation prior to craniosynostosis correction75063). Further study is clearly needed. Meanwhile, there is less controversy when Chiari malformation is associated with hydrocephalus or intracranial hypertension. It is generally agreed that hydrocephalus or intracranial hypertension should be resolved before the Chiari decompression or craniosynostosis correction.
BRAIN PARENCHYMAL MALFORMATION IN CRANIOSYNOSTOSIS
Currently, the genetic understanding of craniosynostosis is remarkably increasing. Various genetic mutations related to craniosynostosis are identified, e.g., mutations of FGFR, TWIST, MSX2, and EFNB1 gene. Mutations of these genes can also be associated with the different phenotypes of the brain parenchyma as well as skull deformity. Non-progressive ventriculomegaly, agenesis of the corpus callosum, defects of the septum pellucidum and mesial temporal abnormalities are typical parenchymal abnormalities in syndromic craniosynostosis45).
Phenotypes of brain malformation may vary widely depending on the type of syndrome. For instance, Apert syndrome could accompany with the absence of olfactory bulbs and tracts, midline fusion of olfactory tubercles, incomplete development of hippocampus, abnormal pyramidal tracts3545), abnormal corpus callosum8913), the septum defects13), gyral abnormalities, grey matter heterotopia, hypoplastic white matter and megalencephaly89). In Crouzon syndrome, hypoplasia or agenesis of the corpus callosum and non-progressive ventriculomegaly is common45).
The exact molecular genetic pathway in these abnormalities is not fully understood, currently. Molecular pathogenesis between genetic mutation and brain parenchymal abnormalities in craniosynostosis are expected to be elucidated in the near future.
NEUROPSYCHOLOGICAL DEVELOPMENT IN CRANIOSYNOSTOSIS
Craniosynostosis, particularly in the syndromic cases, may have the risk of central nervous system damage and associated cognitive impairment by the mechanisms of raised ICP, hydrocephalus and brain parenchymal malformations12). Therefore, there is strong evidence that intellectual and developmental disability occur with greater frequency especially in case with syndromic craniosynostosis than in the normal population40). In an intelligence quotient (IQ) assessment study, children with syndromic craniosynostosis show significantly lower intelligence (mean full scale IQ, 83.1) than normative population averages (mean IQ, 100)12). Despite some argument, there is growing evidence that even non-syndromic single suture synostosis is associated with mild but persistent neuropsychological deficits in a significant number of children3154).
The surgical role in neuropsychological development is still inconclusive. However, considerable studies show that surgical intervention could improve the neurocognitive and behavioral function of patients with craniosynostosis even if they are older than 4 years3032). Furthermore, another study shows that some patients with mild form of craniosynostosis rapidly improve in both or either motor and mental development after cranial expansion52). Therefore, most investigators agree that cranial reconstruction has a positive effect on neuropsychological development in children with craniosynostostosis.
CONCLUSION
Craniosynostosis has a higher clinical significance since it can lead to not only skull deformity but also diverse neurosurgical problems. Moreover, the pathogenesis of these neurosurgical problems is often of multifactorial and even unclear origin. However, finding the exact pathogenesis for each patient could facilitate proper management. Surgery may not offer a cure for this disorder; however, proper neurosurgical management at the appropriate time would ensure excellent outcomes for craniosynostotic patients.
