Malignant Glioma with Neuronal Marker Expression : A Clinicopathological Study of 18 Cases
Article information
Abstract
Objective
Malignant gliomas with neuronal marker expression (MGwNM) are rare and poorly characterized. Increasingly diverse types of MGwNM have been described and these reported cases underscore the dilemmas in the classification and diagnosis of those tumors. The aim of this study is to provide additional insights into MGwNM and present the clinicopathological features of 18 patients.
Methods
We reviewed the medical records of 18 patients diagnosed as MGwNM at our institute between January 2006 and December 2012. Macroscopic total resection was performed in 11 patients (61%). We evaluated the methylation status of O6-methylguanine-DNA methyltransferase (MGMT) and expression of isocitrate dehydrogenase 1 (IDH-1) in all cases, and deletions of 1p and 19q in available cases.
Results
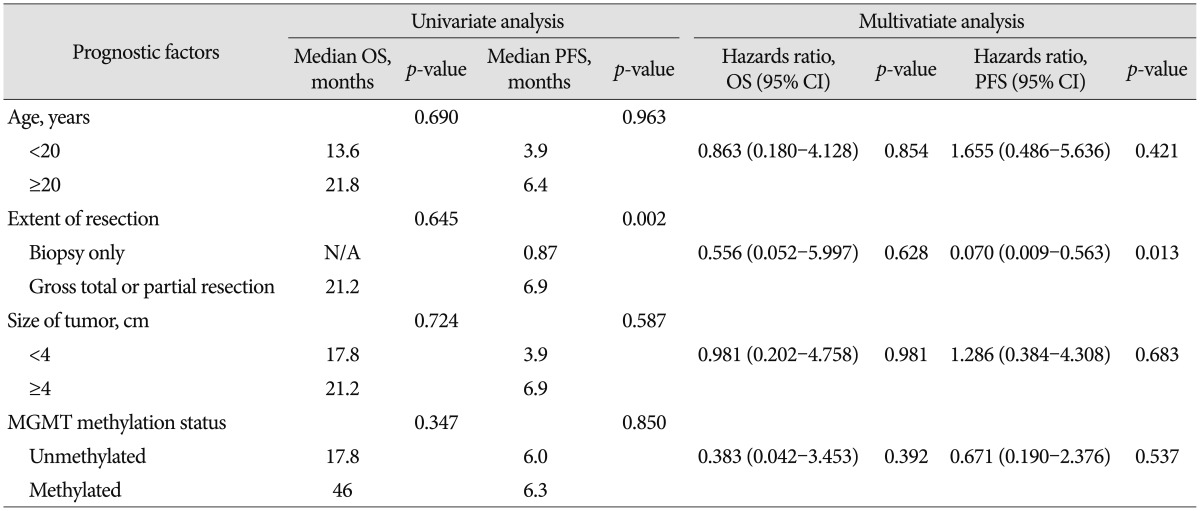
The estimated median overall survival was 21.2 months. The median progression-free survival was 6.3 months. Six patients (33%) had MGMT methylation but IDH1 mutation was found in only one patient (6%). Gene analysis for 1p19q performed in nine patients revealed no deletion in six, 19q deletion only in two, and 1p deletion only in one. The extent of resection was significantly correlated with progression free survival on both univariate analysis and multivariate analysis (p=0.002 and p=0.013, respectively).
Conclusion
In this study, the overall survival of MGwNM was not superior to glioblastoma. The extent of resection has a significant prognostic impact on progression-free survival. Further studies of the prognostic factors related to chemo-radio therapy, similar to studies with glioblastoma, are mandatory to improve survival.
INTRODUCTION
Mixed neuronal-glial tumors, such as ganglioglioma, were first reported in 19307). Anaplastic ganglioglioma is a World Health Organization (WHO) grade III neoplasm, which is defined as a tumor with well-differentiated ganglion cell elements and a histologically malignant glial component5). However, malignant gliomas with neuronal marker expression (MGwNM) other than classical anaplastic ganglioglioma are rare and poorly characterized1422). In recent years, increasingly diverse types of MGwNM have been described. These cases underscore the dilemmas in the classification and diagnosis of MGwNM. It is not clear whether all of these neoplasms are distinct entities, variants or simply histological patterns of the same entity21). Varlet et al.22) reported that gross total surgical resection of MGwNM is an independent and significant predictor of suvival. However, these authors did not analyze adjuvant treatments, such as radiotherapy or chemotherapy, because of the variety of treatment methods. Takeuchi et al.19) resected all tumors followed by radiotherapy and various regimens of chemotherapy in seven patients, but did not mention the treatment-related prognosis.
There are few practical management strategies for this category. These comprise conventional chemotherapy using procarbaxine, lomustinem and vincristine (PCV) and/or radiotherapy. The present study sought to clarify the prognosis and possible factors of MGwNM through a retrospective analysis.
MATERIALS AND METHODS
Study design
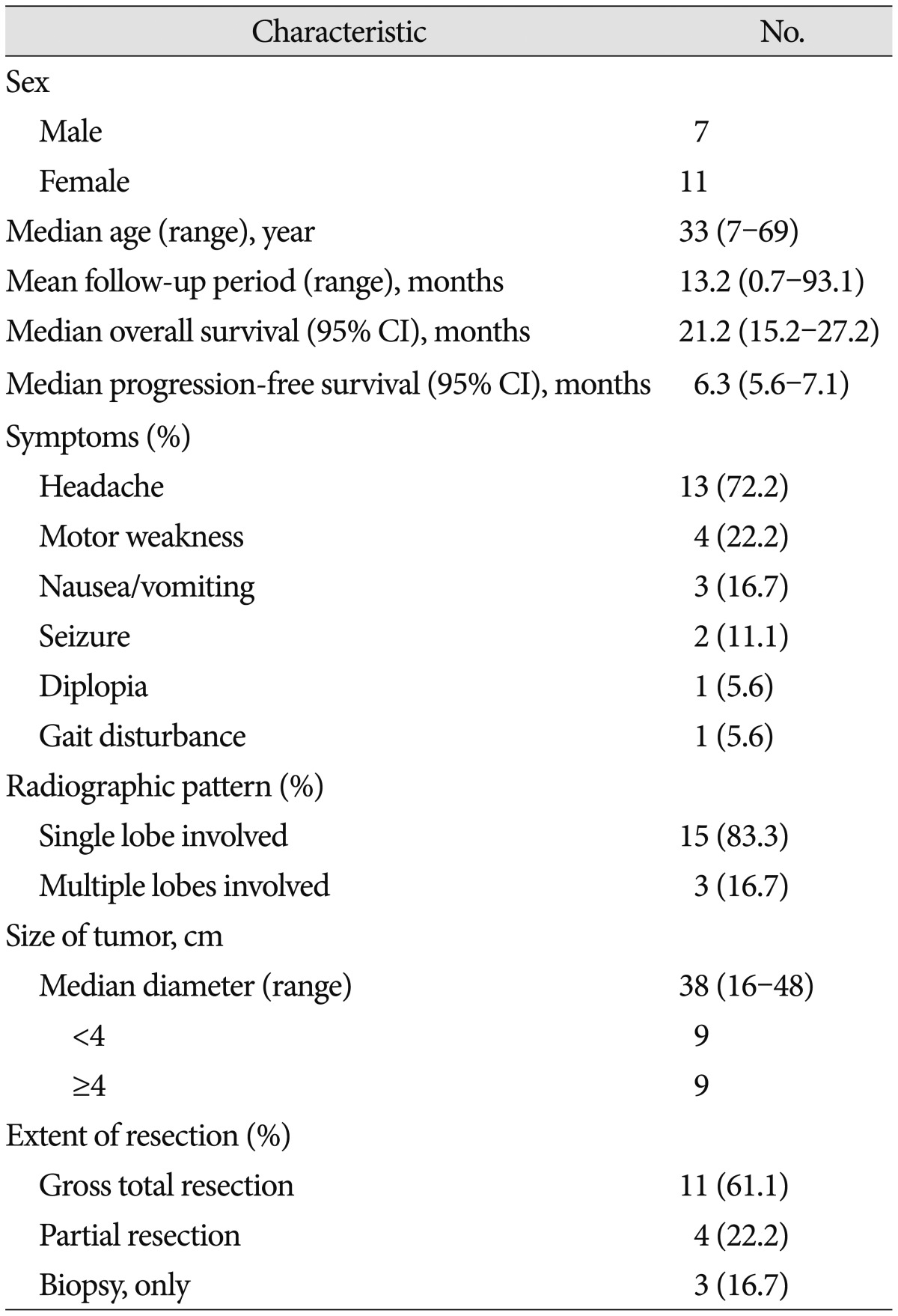
A retrospective review of the medical records of 18 patients who were histopathologically diagnosed with MGwNM at our institute between January 2006 and December 2012 was done. A total of 976 patients were diagnosed with malignant glioma during that period at our institute, so MGwNMs count for 1.8%. Data about clinical presentation and tumor location were collected from the medical records. The extent of surgical resection was assessed according to the surgeon's notes. The 18 patients comprised 7 males and 11 females 7 to 69 years of age (median 33 years). Six patients were younger than 20 years. The most common symptom of patients was headache (n=13, 72%) followed by motor weakness (n=4, 22%) (Table 1). The majority of lesions were located in the temporal lobe (n=6, 33%), frontal lobe (n=5, 28%) and multiple lobes (n=3, 17%). Multiple lobes included, in order of frequency, frontal and parietal lobes followed by frontal and temporal lobes and parietal lobe, occipital lobe, temporal lobe, contralateral hippocampus and periventricular area. In most cases, magnetic resonance imaging showed strong contrast enhancements (n=14, 78%). Peripheral or rim enhancement was also seen in some cases (n=10, 56%). Calcification or hemorrhage within the lesion was noted in five patients. Cystic component and central necrosis was found in 5 (28%) and 2 (11%) cases, respectively. The median tumor diameter was 38 mm (range, 16 to 48).
Macroscopic total resection was performed in 11 patients (61%), partial resection in four (22%), and biopsy in three (17%). All but 2 patients underwent radiotherapy after surgery. However, 2 others (11%) did not receive radiotherapy because of poor medical condition after surgery. For adjuvant chemotherapy, 6 patients received cisplatin and etoposide with or without vincristine chemoregimen before or after radiotherapy, and one patient received two cycles of temozolomide (TMZ) chemotherapy after progression. The patients' clinical characteristics are presented in Table 1. Patients were followed up clinically and radiologically at a regular interval following after surgery.
The study protocol was reviewed and approved by the institutional review board of Samsung Medical Center (SMC 2013-12-145-001) and adhered to the recommendations for biomedical research involving human subjects of the Declaration of Helsinki (1975). The requirement of informed consent was waived, as the study was based on existing clinical data.
Histologic evaluation and immunohistochemistry
Sections from 10% formalin fixed and paraffin embedded (FFPE) tumor samples were stained with hematoxylin-eosin. All slides from all patients were reviewed by the two neuropathologists to confirm the diagnosis of MGwNM (Fig. 1A). Histologically, all the tumors consisted of areas with high grade astrocytoma (WHO grade III or IV) or mixed oligoastrocytoma and the presence of immunoreactive cells for at least one neuronal maker either synaptophysin or neurofilament. A representative section from each patient was selected for further analysis including molecular examination. Immunohistochemistry was performed using a BenchMark XT® autoimmunostainer (Ventana Medical Systems, Tucson, AZ, USA). Antibodies used were against glial fibrillary acidic protein (GFAP, 1 : 4000, Biogenex, Fremont, CA, USA), S-100 (1 : 5000, Dako, Glostrup, Denmark), synaptophysin (1 : 100, Dako, Glostrup, Denmark), phosphorylated neurofilament (1 : 800, Dako, Glostrup, Denmark), neuronal nuclear antigen (NeuN, 1 : 400, Chemicon, Temecula, CA, USA), and isocitrate dehydrogenase 1 (IDH-1, 1 : 50, Dionova, Hamburg, Germany). Strong cytoplasmic staining in any cell was scored as positive. Appropriate positive and negative controls were included.
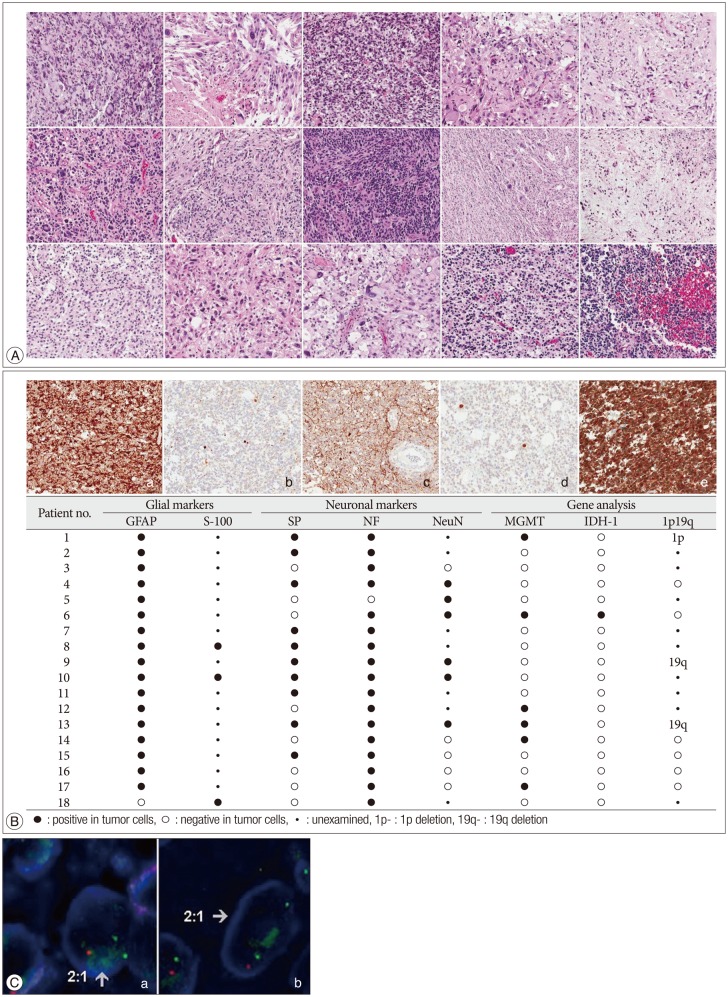
Histologic, immunohistochemical and gene analysis of malignant glioneuronal tumor. A : Hematoxylin-eosin slides of fifteen cases show high grade glial tumor with neuronal component. B : Positive immunohistochemical stains of GFAP, synaptophysin, neurofilament, NeuN, and IDH-1 (a : GFAP, b : synaptophysin, c : neurofilament, d : NeuN, e : IDH-1) and the result of immunohistochemical and gene analysis of 18 patients with malignant glioneuronal tumors. C : Loss of chromosomes 1p and 19q by fluorescence in situ hybridization by using dual color probes (Vysis®LSI®1p36/1q25 and 19q13/19p13). a : two green 1q25 signals and 1 orange 1p36 signal, b : two green 19p13 signals and 1 orange 19q13 signal. NeuN : neuronal nuclear antigen, IDH-1 : isocitrate dehydrogenase 1, GFAP : glial fibrillary acidic protein.
Fluorescence in situ hybridization for 1p and 19q
Fluorescence in situ hybridization (FISH) was performed using dual color probes (Vysis®LSI® 1p36/ 1q25 and 19q13/19p13). Briefly, representative 4 µm thick sections of FFPE tumor tissues were deparaffinized, dehydrated, immersed in 0.2 N HCl, boiled in a microwave in citrate buffer (pH 6.0) and incubated in 1 M NaSCN for 35 minutes at 80℃. Sections were then immersed in pepsin solution, and the tissues were fixed in 10% neutral-buffered formalin. The probe was applied and the sections were appropriately covered and sealed. The slides were incubated in a humidified atmosphere in a Hybrite apparatus (Vysis, Downers Grove, IL, USA) at 73℃ for 5 minutes and 37℃ for 19 hours followed by immersion in 0.4× SSC/0.3% NP-40 at room temperature for 5 minutes and at 73℃ for 5 minutes. After drying, nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI). FISH signals for each locus-specific FISH probe were assessed by examination using a BX51TRF microscope (Olympus, Tokyo, Japan) equipped with a triple-pass filter (DAPI/Green/Orange; Vysis). At least 200 non-overlapping nuclei with intact morphology were evaluated. Deletion was defined as a mean red-to-green signal ratio <0.8.
O6-methylguanine-DNA methyltransferase promoter methylation analysis by quantitative real-time methylation-specific PCR
FFPE DNA was treated with sodium bisulfite using an EZ DNA methylation kit (Zymo Research, Irvine, CA, USA). Methylation specific real-time PCR assays were done in a 7900HT fast real-time PCR system (Applied Biosystems, Foster City, CA, USA). Primer pairs used were O6-methylguanine-DNA methyltransferase (MGMT) forward, CGTTTCGACGTTCGTAGGT and reverse, AAAACTCCGCACTCTTCCG, with TaqMan probe 6FAMAACGACCCAAACACTCACCAAATCGC-BBQ; ACTB forward, TGGTGATGGAGGAGGTTTAGTAAGT and reverse, AACCAATAAAACCTACTCCTCCCTTAA, with TagMan probe 6FAM-ACCACCACCCAACACACAATAACAAACACA-BBQ. The β-actin gene (ACTB) was used to normalize the methylation-independent control reaction. For relative quantification, the amount of methylated DNA (percentage of methylated reference, PMR) at the MGMT promoter region was normalized to the methylation value of the calibrator, which was defined as 100%. Universal methylated DNA (Qiagen, Hilden, Germany) was used as the calibrator. PMR was defined as 100× 2[sampleACTB(ct) - sampleMGMT(ct)]/2[calibrator ACTB(ct) - calibratorMGMT(ct)]. A methylation level ≥3 and <3 was considered methylated and unmethylated, respectively.
Statistical analyses
Overall survival (OS) was defined as the time from the date of first operation to death. Progression free survival (PFS) was defined as the time after the first operation during which patients does not have events including progression of the disease, leptomeningeal seeding, tumor recurrence, metastasis or death. Prognostic and outcome variables associated with OS and PFS in MGwNM that were age, size of tumor, extent of resection and MGMT methylation status in tumor cells. The analysis of the relation between factors mentioned above and OS was calculated by using the Kaplan-Meier method (SPSS version 11.0; SPSS Inc., Chicago, IL, USA). The differences between the survival curves were tested using the log-rank test. The Cox proportional hazards model was used for the multivariate comparisons of median OS and PFS. The results were statistically significant if a 2-sided p value was <0.05.
RESULTS
Clinical outcome
The median follow-up period at the time of analysis was 13.2 months (range, 0.7 to 93.1), and the estimated median OS was 21.2 months [95% confidence interval (95% CI), 15.2 to 27.2]. At the end of follow-up, three (17%) patients remained free of disease progression. The median PFS was 6.3 months (95% CI, 5.6 to 7.1).
Immunohistochemical and gene analysis
Immunohistochemical and gene analysis results are summarized in Fig. 1B. GFAP immunohistochemistry was performed in each patient. Seventeen patients (94%) displayed GFAP-expressing tumor cells. S-100 protein immunohistochemistry was performed in three tumors and strong S-100 protein immunoreactivity was always noted including the GFAP-negative tumor. The neuronal markers NF and SP were examined in all patients and NeuN in 11 patients. Seventeen patients (94%) displayed NF-positive tumor cells and 10 patients (56%) were SP positive. Six (55%) patients in whom NeuN immunostaining was performed were positive. All patients showed positive immunostaining results at least in one of the immunostaining examinations. IDH-1 was positive in only one patient (6%). MGMT methylation was evaluated in all 18 patients and detected in 6 patients (33%). Patients who underwent TMZ chemotherapy did not present methylated MGMT. 1p19q FISH performed in 9 patients (Fig. 1C) revealed no deletion for 1p or 19q in 6 patients, 19q deletion only in 2 patients and 1p deletion only in the remaining patient.
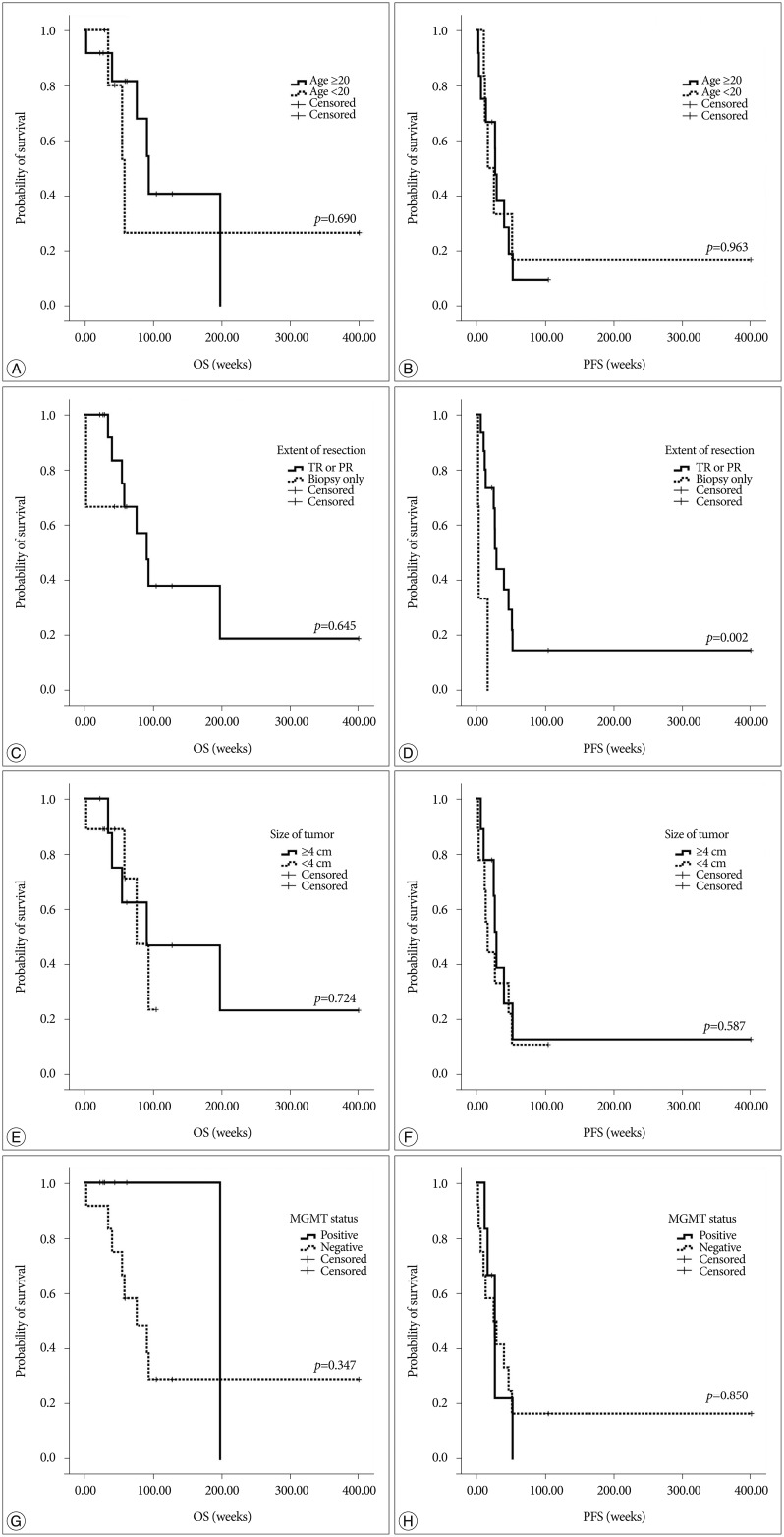
On univariate analysis, age, size of tumor, extent of resection and MGMT methylation status in tumor cells were not correlated with OS. Only extent of resection (gross total resection or partial resection versus biopsy only) was correlated with PFS (p=0.002) (Table 2, Fig. 2). On multivariate analysis, extent of resection (biopsy, only) was found to be the most significant independent predictors of poor PFS (p=0.013) (Table 2).
DISCUSSION
Since Varlet et al. suggested MGwNM as a distinct entity some cases have been reported to fall under the category171922). Nonetheless, those tumors have until recently remained poorly characterized and its epidemiology is still not well known. High-grade gliomas are significantly less common in children than adults and represent approximately 6.5% of all newly diagnosed childhood intracranial neoplasms20). Presently, MGwNM occurred both in adults (n=12, 67%) and children (n=6, 33%) although there was no significant prognostic value according to age. Patients who were treated with gross total or partial surgical resection had a more favorable prognostic impact in PFS than who underwent biopsy only, whereas there was no significantly correlated value in OS.
To the best of our knowledge, only limited series of patients with MGwNM have been reported. Treatment options that include surgical resection extent, radiation therapy and chemotherapy are varied and unstandardized. Moreover, the results of each treatment are too heterogeneous and it is impossible to assume the OS or PFS (Table 3)12481316192122). Although those tumors generally have a more favorable prognosis than glioblastomas22), the present median OS of 21.2 months and PFS of 6.3 months of patients with the pathological diagnosis were not so favorable.
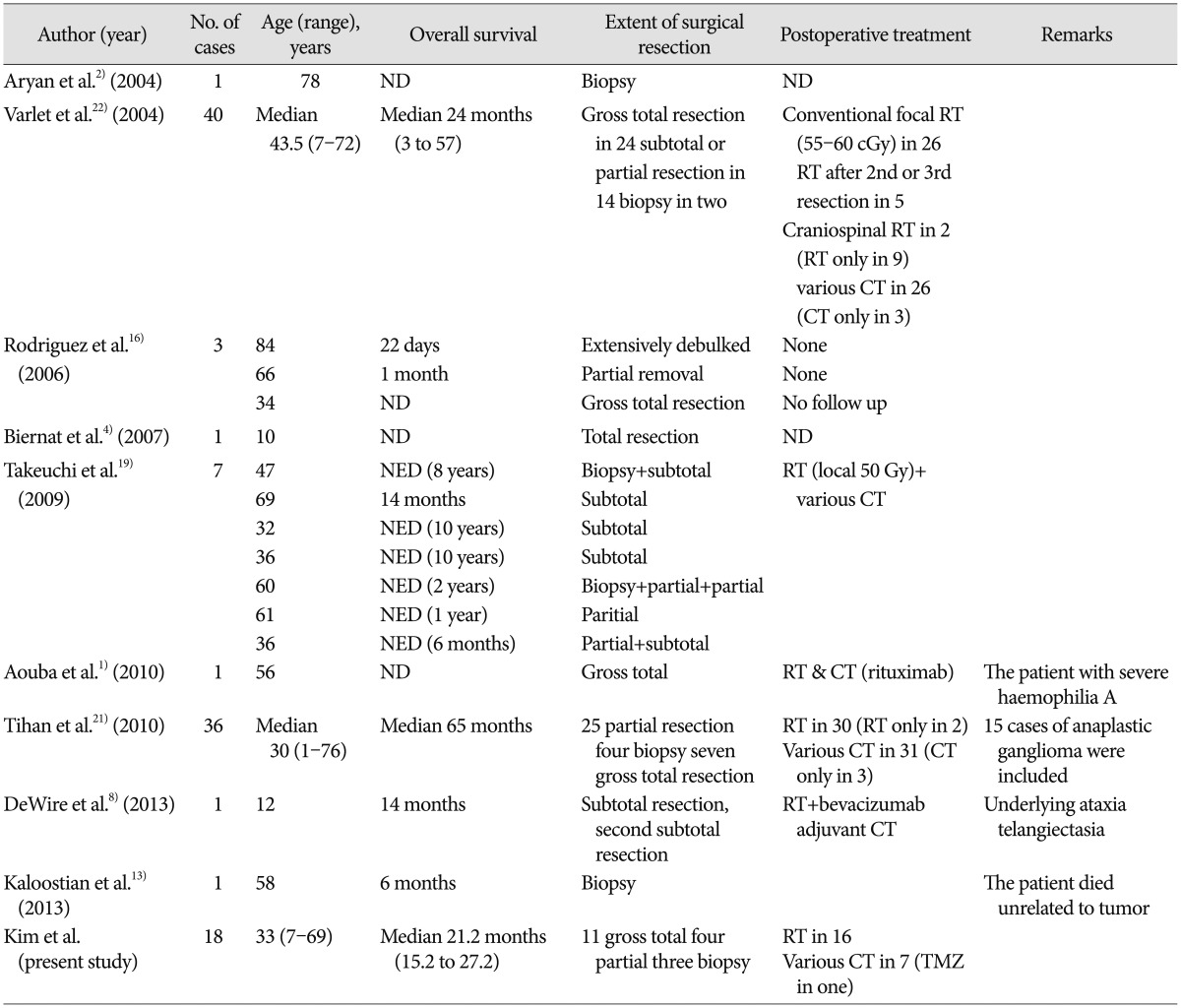
Comparison of patient overall survival and treatment options of the previously reported cases of malignant glioneuronal tumors
MGwNM in this study were distinguished from anaplastic gangliogliomas by the lack of atypical ganglion cells. All tumors exhibited glial differentiation that was positive for glial markers (either GFAP or S-100).
Three markers including 1p19q deletion, MGMT methylation and IDH-1 mutation are considered as indicators of favorable prognosis in malignant gliomas691011121523). Our results demonstrated the status of these markers in all 18 patients with MGwNM.
Takeuchi et al.19) reported deletions of chromosome 1p19q in seven patients and suggested that 1p19q loss and differentiation along neuronal cell lines as a factor in the favorable prognosis for glial tumors. Presently, there were also3 patients (17%) with deletion in 1p (n=1) or 19q (n=2). However, both 1p and 19q deletions were not detected in any patient, so further analysis with 1p19 deletion was not performed.
MGMT promoter methylation is associated with a favorable outcome after alkylating chemotherapy with TMZ in patients with newly diagnosed glioblastoma91011). The MGMT gene is located on chromosome 10q26 and encodes a DNA-repair protein that removes alkyl groups from the O6 position of guanine, an important site of DNA alkylation11). Our molecular genetic analysis showed that methylated MGMT were also identified in MGwNM. In this study, only one patient received TMZ chemotherapy; MGMT methylation was not detected in the tumor cells of this patient. There were six other patients with methylated MGMT status in which no significant correlated prognostic value was evident. This result underlines the need of trying conventional chemotherapy, such as TMZ, as an addition to surgical resection. No standard chemotherapy for this category is known and no investigation has addressed TMZ chemotherapy in patients with those tumors. Our study indicates that attempting TMZ chemotherapy in patients with MGwNM may be prudent, especially when methylated MGMT is detected.
As far as we know, the current study is the first investigation of the IDH-1 status in patients with MGwNM. In 2008, a genome-wide somatic mutational analysis of glioblastoma revealed a subset of an IDH-1 mutation at codon R13215). Subsequently, IDH-1 mutations ware detected in all subtypes of gliomas, with the exception of primary glioblastomas (5–8%)323). In our study, IDH-1 mutation was detected in only one case of MGwNM so further analysis could not be undertaken. However, the analysis of IDH-1 in more patients with those tumors may be needed to identify the prognostic factors and set new options for treatment of MGwNM.
Our study has 2 limitations. First, we failed to validate the effect of conventional treatments for malignant gliomas such as TMZ chemotherapy followed with CCRT, because it was difficult to try TMZ chemotherapy due to lack of objective evidence and practical cost-effectiveness. Second, we could not test 1p19q in all patients as the non-availability of samples or absent of informed consent, so we could not analyze the prognosis according to the 1p19q deletion.
Nonetheless, the results show some possibility that the prognosis of MGwNM is not so favorable than glioblastomas. Confirmation in more patients is recommended. In addition, the extent of resection has a significant prognostic impact in PFS value. Those are important clinical findings to determine optimal management for these tumors. Establishing decisive pathological differential points of anaplastic ganglioneuronal tumors or neural type glioblastomas is prudent. Conventional treatment for glioblastomas seems warranted considering the poor prognosis of MGwNM, particularly in patients with MGMT methylated MGwNM. Additional gene analyses, such as 1p19q deletion and IDH-1 mutation, could give more information about prognostic factors of this category tumors. A better understanding of clinically feasible prognostic factors regarding chemo- or radiation therapy could lead to more effective treatments of those tumors.
CONCLUSION
In this study, the prognosis of MGwNM was not superior to glioblastoma. The extent of resection has a significant prognostic impact on progression-free survival. Further studies of the prognostic factors related to chemo-radio therapy, similar to studies with glioblastoma, are mandatory to determine optimal treatment and improve survival.
Acknowledgements
This work was equally supported by the Global Frontier Project grant (NRF-2012M3A6A-2010-0029781) of National Research Foundation funded by the Ministry of Education, Science and Technology of Korea and by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C3418).