Intracranial Meningiomas, WHO Grade Il : Prognostic Implications of Clinicopathologic Features
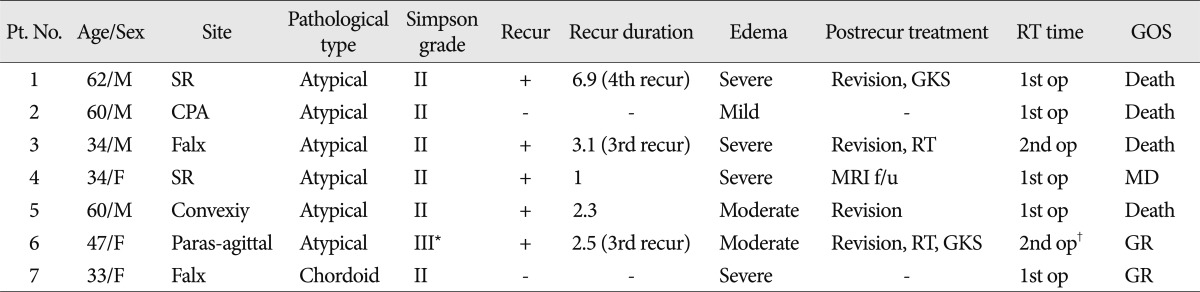
Article information
Abstract
Objective
Intracranial meningiomas are primarily benign tumors with a good prognosis. Although WHO grade II meningiomas are rare (2-10%), WHO grade II meningiomas have higher recurrence and mortality rates than benign. We evaluated the patient recurrence rate and investigated the prognostic factors of WHO grade II meningiomas.
Methods
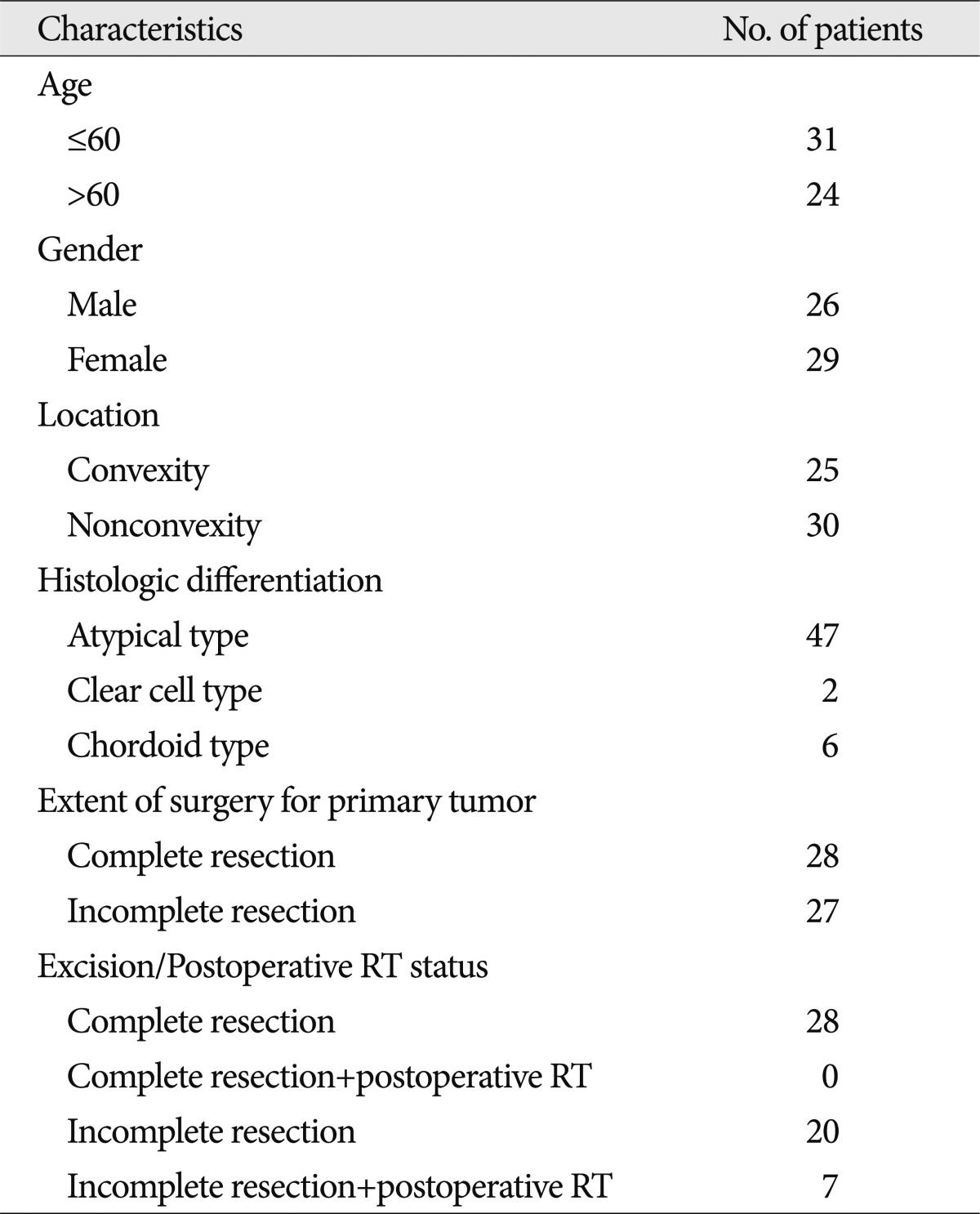
Between 1993 and 2005, 55 patients were diagnosed with WHO grade II meningiomas in our hospital. WHO grade II meningiomas (n=55) were compared with other WHO grades meningiomas (I, n=373; and III, n=20). The patients had a median age of 48.4 years (range, 14-17 years), a male-to-female ratio of 26 : 29, and a mean follow-up time of 45 months (range, 3-175 months).
Results
In WHO grade II meningiomas, only the extent of resection was a significant prognostic factor. Post-operative radiotherapy had no significant influence on tumor recurrence (p=0.053). The relative risk of recurrence was significantly higher in WHO grade II meningiomas with incomplete resection (10/27, RR=37%) than in WHO grade II meningiomas with complete resection (4/28, RR=14%) regardless of post-operative radiotherapy. In the incomplete resection group, Simpson grade III or IV had a significantly high risk of recurrence regardless of post-operative RT (n=3, RR=100%) However, if the degree of resection was Simpson grade II, the recurrence rate was similar to the complete resection group even though post-operative RT was not performed.
Conclusion
Complete resection was the most powerful independent predictive factor of the recurrence rate in WHO grade II meningiomas. Post-operative adjuvant RT was not a significant factor in this study.
INTRODUCTION
Meningiomas constitute approximately 20% of all brain tumors8). In 1922, Harvey Cushing first used the term meningioma16). According to the World Health Organization (WHO) histologic grading system, three grades of meningiomas with increasing risks of recurrence are distinguished8). Benign or common-type tumors with a low rate of recurrence (7-20%) are assigned to grade I. Grade II meningiomas include atypical meningiomas as well as the rare chordoid and clear cell (intracranial) variants, and exhibit a higher risk of recurrence (29-40%). Grade III meningiomas are anaplastic meningiomas with high mitotic activity (≥20/10 high-power fields) and/or obviously malignant cytology, as well as the rare variants, papillary and rhabdoid meningiomas. The recurrence rate of anaplastic meningiomas is suggested to be 50-78%. Whereas a retrospective review showed that resections of WHO grade II meningiomas are less often classified by surgeons as gross total when compared with benign meningiomas, a majority of WHO grade II meningiomas are still able to undergo gross total resection (GTR) as the initial treatment17). The subsequent prognosis and optimal management after GTR of WHO grade II meningiomas remain unclear. We sought to define the long-term recurrence rate and predictive factors for recurrence of WHO grade II meningiomas.
MATERIALS AND METHODS
Patient selection
Between 1993 and 2005, 448 patients with an intracranial meningioma were treated surgically at our institution by one surgeon. We retrospectively reviewed records of the 55 patients with intracranial WHO grade II meningiomas. The mean duration of follow-up was 45 months (range, 3-175 months). Twenty-eight tumors had undergone Simpson grade I (complete) resection, as confirmed by both the surgeon's impression at the time of surgery and the first post-operative imaging scans. This study was approved by the Institutional Review Board at our institution. The patient and tumor characteristics of 55 WHO grade II meningiomas are summarized in Table 1.
Parameters assessed
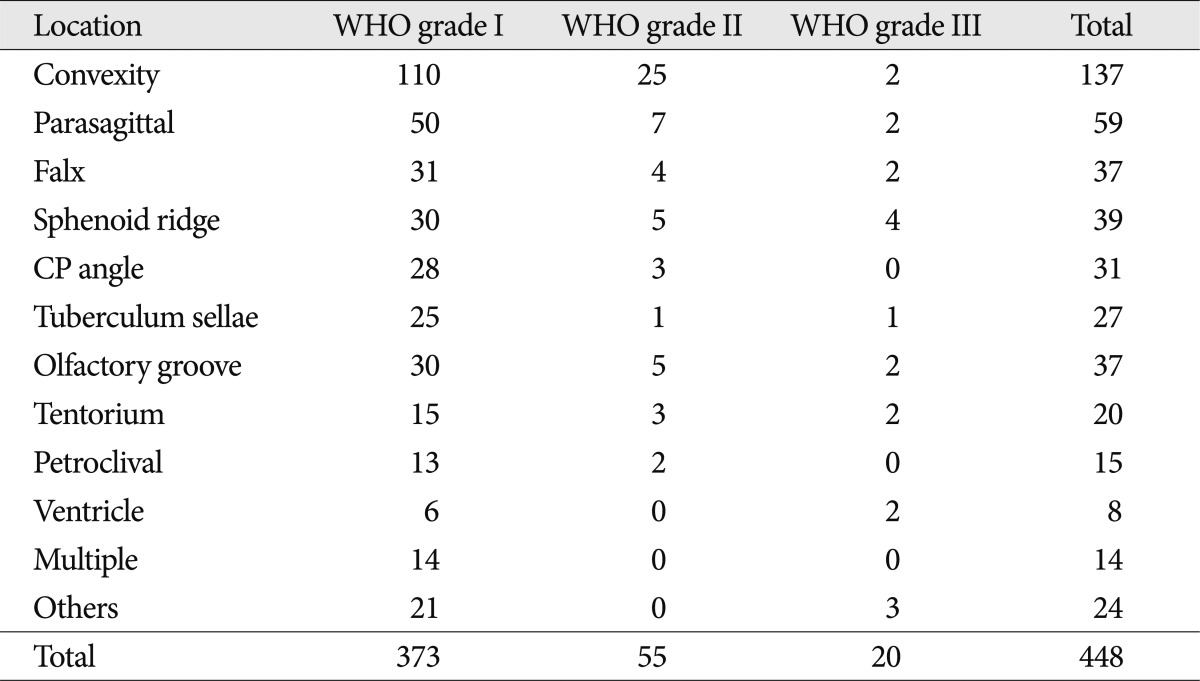
Age at the time of diagnosis was defined as the patient's age at the time of his or her first surgery. Tumor size was defined by the largest tumor diameter rounded to the nearest centimeter on imaging scans before the initial surgery. The tumors were sub-grouped according to location, as follows : convexity; parasagittal; falx; sphenoid ridge; cerebellopontine angle; tuberculum sellae; olfactory groove; and tentorium (Table 2). "Complete resection" is defined as Simpson grade I, and "incomplete resection" is defined as Simpson grade II, III, or IV. The meningiomas were also divided into groups, as follows : convexity and non-convexity groups. The anatomic distribution of the 55 meningiomas that were totally resected during this period differed in having a higher proportion of tumors in the "convexity" group (19 of 25 patients) and a lower proportion in the "non-convexity" group (9 of 30 patients).
Surgical strategy
Patients with completely resected WHO grade II meningiomas who do not receive radiation therapy are observed closely in our institution. We recommend post-operative radiation therapy in patients with grade II meningiomas which are not completely resected. In recurrent cases, reoperation should be considered first. Gamma knife radiosurgery is an alternative treatment modality in small or surgically inaccessible meningiomas or meningiomas, and patients of advanced age and poor operative risk.
Statistical analysis
Recurrence-free rates were estimated using the Kaplan-Meier method. Logistic regression, Mantel-Cox, and Breslow-Gehan-Wilcoxon univariate and multivariate models were used to compare the recurrence-free survival distributions across subgroups. The following variable data were analyzed by multiple regression analysis with recurrence as the dependent variable : age; gender; tumor size; peritumoral edema; extent of tumor removal; and tumor location. Values are expressed as the means+standard deviation. The level of statistical significance was regarded at p values <0.05.
RESULTS
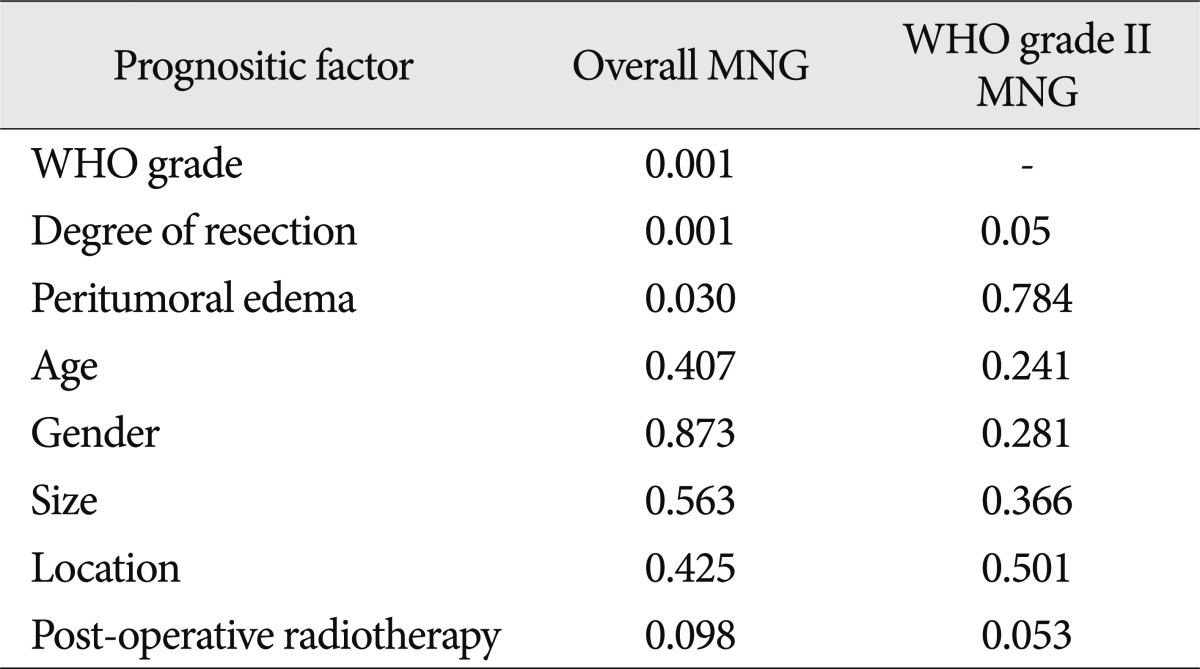
Of the 448 patients, 20 had WHO grade III meningiomas, 55 had WHO grade II meningiomas, and 373 had benign meningiomas. Table 2 shows the number of patients followed with each WHO grade of meningioma. A multivariate analysis was then performed to assess the following eight variables as possible prognostic factors for tumor recurrence : 1) age; 2) gender; 3) tumor location; 4) tumor size; 5) peritumoral edema; 6) post-operative radiation therapy; 7) histologic type; and 8) extent of resection. Of these eight factors, three were predictive of recurrence based on multivariate analysis, as follows : pathologic subtype (p=0.001); degree of resection (p=0.001); and peritumoral edema (p=0.016). The extent of resection was a significant independent predictive factor for WHO grade II meningiomas (p=0.05) (Table 3).
Of the 55 patients diagnosed with WHO grade II meningiomas at our institution between 1993 and 2005, 26 were men and 29 were women. The mean age at the time of diagnosis was 55 years (range, 19-82 years). Clinical follow-up included serial imaging for an average of 45 months after tumor resection (range, 3-175 months) with 40% of the patients followed either to recurrence or for at least 5 years after surgery.
In WHO grade II patients, 14 had recurrences 7-96 months post-operatively; 11 patients had recurrences 5 years post-operatively (mean time to recurrence, 42.9 months), and 3 had recurrences 10 years post-operatively. Complete resection was achieved in 28 patients and incomplete resection was achieved in 27 patients. Post-operative adjuvant radiotherapy was delivered in 7 patients after incomplete resection.
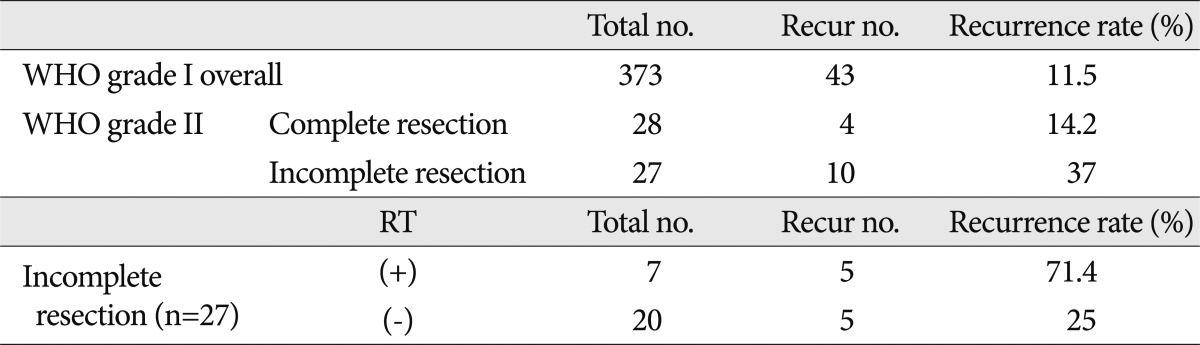
The overall recurrence rate for WHO grade II meningiomas was 25.5% (n=55). The recurrence rate between the two groups of 'WHO grade II meningioma patients (complete resection)' and 'WHO grade I meningioma patients (complete or incomplete resection)' were 14.2% and 11.5%, respectively (Table 4, Fig. 1, 2C). Although it was difficult to show statistical significance, the recurrence rates were similar between the benign meningioma treatment group and the WHO grade II meningioma without radiotherapy treatment group (p=0.844). However, the relative risk (RR) of recurrence was significantly higher for patients with WHO grade II meningiomas with incomplete resections (10/27, RR=37%) than patients with complete resections (4/28, RR=14%), independent of post-operative radiotherapy. Fig. 2B represents the recurrence-free survival between the two treatment groups (p=0.05).
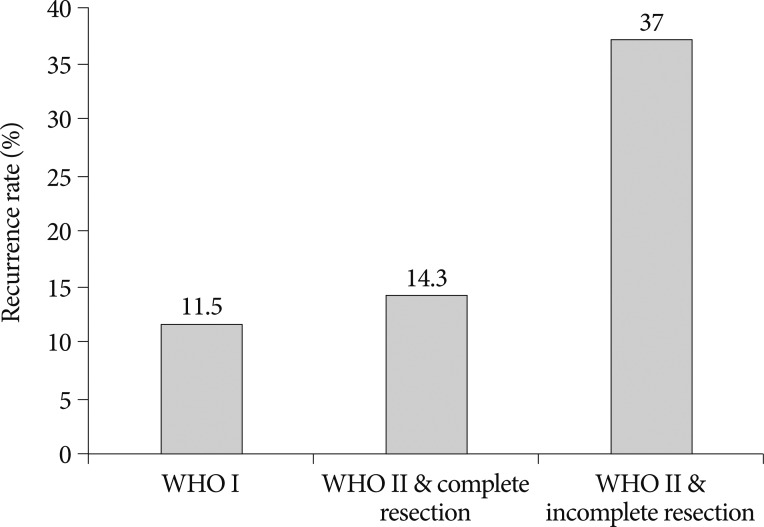
The recurrence rates are similar between the benign meningioma and WHO grade II meningioma complete resection treatment groups. However, the RR is significantly higher for patients with WHO grade II meningiomas with incomplete resection than patients undergoing complete resection. WHO : World Health Organization, RR : relative risk.

Kaplan-Meier recurrence-free survival analysis in patients with meningiomas. A : Recurrences between the pathologic types. The survival curves are significantly better for patients with WHO grade I compared with WHO grade II or III (p=0.001). B : The risk of recurrence is significantly higher with the incomplete resection group than the complete resection group in WHO grade II meningiomas (p=0.05). C : The recurrence rate between the two groups of 'WHO grade II patients who had complete resection' and 'WHO grade I overall (complete or incomplete resection)' are similar. D : Degree of peritumoral edema is also a significant prognostic factor in overall meningiomas (p=0.03). WHO : World Health Organization.
In the incomplete resection group, 5 of 20 patients with recurrences did not receive radiotherapy. Five of 7 patients had recurrences after post-operative radiotherapy (Table 4). We analyzed the recurrence-free survival between these two groups, but it was difficult to interpret the statistical significance of the influence of radiotherapy on recurrence (p=0.314). When subdividing this group based on Simpson grade, the Simpson grade III or IV group had a high risk of recurrence regardless of post-operative radiotherapy (n=3, RR=100%). However, if the degree of resection was Simpson grade II, the recurrence rate was similar to the complete resection group, although post-operative radiotherapy was not administered (3/18, RR=15.5%).
Table 5 shows the information regarding patients who did not receive radiotherapy after incomplete resection; five patients had recurrences. This group had distinguishing features from other groups. With the exception of one patient, all patients were in the non-convexity area, and in whom it was more difficult to perform complete resections than in patients in the convexity area. Most of the patients had moderate-to-severe degrees of peritumoral edema; three of the patients had several recurrences. We delivered post-operative radiotherapy to this patients who had more risky conditions for recurrence than other patients.
Anatomically, there were higher proportions of convexity meningiomas in the completely resected group [67% (19/28)] than in the incompletely resected group [22% (6/27)].
Considering the importance of the degree of resection and post-operative radiation therapy, we compared the Simpson grade I resection group without radiotherapy in convexity and Simpson grade II resection followed by post-operative radiotherapy group in non-convexity. The recurrence rate was significantly higher in the latter (60%) than the former (15%).
DISCUSSION
In this study, we sought to define the long-term recurrence rate of WHO grade II meningiomas after complete or incomplete resection, as well as factors influencing recurrence, including post-operative radiation. Thus, we attempted to reveal what clinicopathologic features may be useful for predicting recurrence of WHO grade II meningiomas.
It is well-known that the histologic type is a significant prognostic factor in overall meningiomas. In our study, according to the Kaplan-Meier survival analysis, the recurrence rate between the pathologic types was shown to be lowest in WHO grade I meningiomas and highest in WHO grade III meningiomas (Fig. 2A). The p value was 0.001. According to previous reports, the recurrence rate increased with the histologic grade rising, consistent with the findings of Ayerbe et al.1) Mantle et al.9) reported that meningiomas invade brain tissue along cells, forming bridges between the cortex and surface of the tumor. Mantle et al.9) suggested that the invasive cells were the main reason for tumor recurrence.
Some authors state that tumor size may be helpful in predicting the recurrence of WHO grade II meningioma14). Also, several studies reported that males have higher recurrence rate4,15), and development of tumors in patients at a young age (<40 years) was associated with higher likelihood of recurrence15), although these were not clear in this or other studies7,12).
In the present study, the degree of peritumoral edema is a significant prognostic factor in overall meningiomas, but has no predictability for WHO grade II meningiomas (Fig. 2D, Table 3). Some authors have reported statistical significance as predictive factors for recurrence of meningiomas20) because edema represent a variety of pathologic changes, such as a cerebral-pial vascular supply and cerebral white matter hypoperfusion13,18,20).
The degree of resection as a significant independent predictive factor of recurrence in WHO grade II meningiomas as well as in overall meningiomas, many authors had reported similar results. Mirimanoff et al.11) reported recurrence-free survival rates after total resection of 93% at 5 years, 80% at 10 years, and 68% at 15 years; compared with partial resection, the recurrence-free survival rates dropped to 63%, 45%, and 9%, respectively12). Jaaskelainen5) reported that after complete resection the recurrence rates were 19% at the 20-year follow-up. The same group reported that in patients with atypical or malignant meningiomas after complete resection the risk of recurrence was 38% and 78% at 5 years, respectively6). Goyal et al.3) reported that 8 of 22 patients had local recurrences, including 2 of 15 with GTR, 3 of 4 with STR, and all 3 patients had resection of unknown extent. At 10 years, patients with GTR had a higher local control rate than those who had a STR or a resection of unknown extent (87% vs. 17%; p=0.02). The results of the present study correspond well with those of the earlier study. The most important factors that determine the likelihood of meningioma recurrences is the extent of tumor resection. We proved that through Kaplan-Meier survival analysis (Fig. 2B).
Although location was not a significant predictive factor for recurrence of meningiomas in our study, location is an important factor influencing the extent of resection of meningiomas. Convexity meningiomas are an indication for complete resection, Simpson grade I can also identify complete resection most significantly than other locations. We reasoned that comparing the two groups, Simpson grade I resection without RT in the convexity group and Simpson grade II resection followed by post-operative radiotherapy in the non-convexity group was meaningful to minimize bias. For amplifying the clarity of the relationship between the extent of resection and recurrence considering the potential difficulty of radical resection in non-convexity, we compared two groups to the exclusion of the Simpson grade III or IV group; the recurrence rate of the former was 15.7% and the recurrence rate of the latter was 60%. It was reconfirmed that the extent of resection was a powerful factor for decreasing recurrence of WHO grade II meningiomas.
Post-operative adjuvant radiotherapy after surgical resection of WHO grade II meningiomas continues to be controversial. In our study we could not find statistical significance of the influence of radiotherapy on recurrence. Furthermore, the incompletely resected group with radiotherapy had a higher recurrence rate than the group without radiotherapy. In contrast to our expectations, some patients did well for many years after subtotal resections alone. We presumed the cause of a higher recurrence rate was due to the fact that the patients had been expected to have a worse prognosis because of factors, such as severe edema or complex site or previous recurrence underwent radiotherapy (Table 5).
Most of the authors tend not to recommend adjuvant radiotherapy in grade I meningiomas. If gross total resection was achieved, however, there are significant variations in opinion about the effect of post-operative radiotherapy in cases of completely resected grade II meningiomas10) Therefore, we compared the recurrence rate between two groups (the overall treatment group of benign meningiomas and the complete resection group of WHO grade II meningiomas without radiotherapy). Although it was difficult to show statistical significance (p=0.844), the recurrence rate between the two groups were similar to each other (Table 4, Fig. 2C). This suggests that complete resection of WHO grade II meningiomas reduced the recurrence rate to that of WHO grade I meningiomas, regardless of post-operative radiotherapy. However, in the completely resected group, the effect of post-operative radiation therapy was uncertain in this study.
We subdivided the incomplete resection group through Simpson grade for researching the effect of post-operative radiotherapy. The group performed by Simpson grade III or IV had a significantly high risk of recurrence without reference to post-operative radiotherapy. If the degree of resection is Simpson grade II, the recurrence rate is similar to the complete resection group, although post-operative radiotherapy was not performed. It is questionable whether or not post-operative radiotherapy must be performed in WHO grade II meningiomas after Simpson grade II resection. This study demonstrated the effect of radiotherapy for preventing recurrence is minimal or none.
Some authors have found statistical significance between radiotherapy and the recurrence rate, and they recommended irradiation, irrespective of the extend of resection2,19). However, in another study3) in which 8 of 22 patients with atypical meningiomas received post-operative radiotherapy, local control was 87% at 5 and 10 years following GTR, and radiotherapy had no significant impact on local control or overall survival. McCarthy et al.11) reported studies which included patients with grade II meningiomas who underwent gross total resection, and failed to demonstrate a statistically significant change in local control or survival in patients receiving adjuvant radiotherapy. A number of authors do not recommend radiotherapy for atypical meningiomas regarding the extent of surgical resection because the majority of patients with atypical meningiomas would ultimately experience local recurrence despite a course of conventional radiation treatment5,7) There are no randomized trials to support any opinion regarding the role of post-operative radiotherapy, and it is still debatable whether or not these patients should be carefully observed or treated pre-emptively. Because of the rarity of WHO grade II meningiomas and differences in the way WHO grade II meningiomas are classified, the literature on the role of radiation in treating such patients are difficult to interpret.
This study had a limitation with respect to the small sample size. Also, the data for our study were collected retrospectively, and thus a potential for bias may exist. The gold standard for evaluating our treatment policy is a prospective randomized trial, but this approach is thought to be difficult due to the rarity of atypical meningiomas. Nevertheless, the fact that the surgical procedure was performed by one surgeon adds credibility to our study.
Summary
1) In WHO grade II meningiomas, complete resection (Simpson grade I) is the most important factor for preventing recurrence. However, in the completely resected group, the effect of post-operative radiation therapy is uncertain in this study.
2) If the degree of resection is Simpson grade II, the recurrence rate is similar to the complete resection group, although post-operative radiotherapy is not performed. It is questionable whether or not post-operative radiotherapy must be performed in WHO grade II meningiomas after Simpson grade II resection. This study demonstrates the effect of radiotherapy for preventing recurrence is minimal or none.
3) Regardless of post-operative radiotherapy, if the degree of resection is Simpson grade III or IV, the risk of recurrence is significantly high.
CONCLUSION
The extent of resection is the most powerful independent predictive factor for recurrence of WHO grade II meningiomas. If complete resection was performed, the recurrence rate of WHO grade II meningiomas was reduced to that of benign meningiomas, regardless of adjuvant treatment. Post-operative adjuvant radiotherapy was not a significant factor in this study, also it continued to be controversial according to previous reports. However, because of several limitations of this study, re-estimation of the role of post-operative radiotherapy is required.
Acknowledgements
This study was supported by the Brain Korea 21 Project, Center for Biomedical Human Resources at Chonnam National University. This research was supported by Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST).