Eun and Park: Early Restoration of Hypoperfusion Confirmed by Perfusion Magnetic Resonance Image after Emergency Superficial Temporal Artery to Middle Cerebral Artery Anastomosis
Abstract
Objective
Emergency superficial temporal artery to middle cerebral artery (STA-MCA) anastomosis in patients with large vessel occlusion who fails mechanical thrombectomy or does not become an indication due to over the time window can be done as an alternative for blood flow restoration. The authors planned this study to quantitatively measure the degree of improvement in cerebral perfusion flow using perfusion magnetic resonance imaging (MRI) after bypass surgery and to find out what factors are related to the outcome of the bypass surgery.
Methods
For a total of 107 patients who underwent emergent STA-MCA bypass surgery with large vessel occlusion, the National Institute of Health stroke scale (NIHSS), modified Rankin score (mRS), infarction volume, and hypoperfusion area volume was calculated, the duration between symptom onset and reperfusion time, occlusion site and infarction type were analyzed. After emergency STA-MCA bypass, hypoperfusion area volume at post-operative 7 days was calculated and analyzed compared with pre-operative hypoperfusion area volume. The factors affecting the improvement of mRS were analyzed. The clinical status of patients who underwent emergency bypass was investigated by mRS and NIHSS before and after surgery, and changes in infarct volume, extent, degree of collateral circulation, and hypoperfusion area volume were measured using MRI and digital subtraction angiography (DSA).
Results
The preoperative infarction volume was median 10 mL and the hypoperfusion area volume was median 101 mL. NIHSS was a median of 8 points, and the last normal to operation time was a median of 60.7 hours. STA patency was fair in 97.1% of patients at 6 months follow-up DSA and recanalization of the occluded vessel was confirmed at 26.5% of patients. Infarction volume significantly influenced the improvement of mRS (p=0.010) but preoperative hypoperfusion volume was not significantly influenced (p=0.192), and the infarction type showed marginal significance (p=0.0508). Preoperative NIHSS, initial mRS, occlusion vessel type, and last normal to operation time did not influence the improvement of mRS (p=0.272, 0.941, 0.354, and 0.391).
Conclusion
In a patient who had an acute cerebral infarction due to large vessel occlusion with large ischemic penumbra but was unable to perform mechanical thrombectomy, STA-MCA bypass could be performed. By using time-to-peak images of perfusion MRI, it is possible to quickly and easily confirm that the brain tissue at risk is preserved and that the ischemic penumbra is recovered to a normal blood flow state.
Key Words: Cerebrovascular disorders · Cerebral revascularization · Perfusion imaging.
INTRODUCTION
The role of intra-arterial thrombectomy in cerebral large vessel occlusion has been increased [ 1, 15]. As imaging modalities for the collateral circulation and endovascular devices for mechanical thrombectomy have been improved, applicable patients for mechanical thrombectomy were expanded. In a recent report, the recanalization rate of mechanical thrombectomy for large vessel occlusion was about 74% [ 19]. However, many patients cannot undergo mechanical thrombectomy due to various reasons such as low National Institute of Health stroke scale (NIHSS) or late hospital visit time. For these patients, surgeries for augmentation of cerebral blood flow (CBF) are becoming alternatives. Superficial temporal artery to middle cerebral artery (STA-MCA) anastomosis is one of the alternatives. Although the efficacy of emergent STA-MCA anastomosis has been reported [ 10, 11, 16], there are concerns about the effectiveness of STA-MCA anastomosis because STA-MCA anastomosis is a lowflow bypass surgery that only restoring a little blood flow. In this study, we analyzed the effectivity of STA-MCA anastomosis for improving perfusion defects after large vessel occlusion by perfusion magnetic resonance imaging (MRI) and which factors were associated with the improvement of the patient’s postoperative outcome.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board (IRB) of The Catholic University of Korea (HC21RISI0091). The IRB approved a request to waive the informed consent. From May 2012 to January 2020, patients who underwent emergent STA-MCA bypass surgery due to acute cerebral infarction with large cerebral vessel occlusion were enrolled with a protocol, and their clinical features and imaging data were recorded. The clinical features of all patients were evaluated by neurological examination, including motor weakness, degree of speech impairment, modified Rankin score (mRS), and NIHSS before and immediately after surgery and at postoperative 1 month and 6 months. Computed tomography (CT) angiography, diffusion MRI, perfusion MRI, and digital subtraction angiography (DSA) were evaluated before and after surgery. NIHSS was evaluated at the emergency room and evaluated again before bypass surgery if symptoms progressed. Infarction volume was measured based on diffusion images before surgery, and the vessel occlusion site was evaluated with DSA. Hypoperfusion area volume is defined as indicated in red color on the time-to-peak (TTP) map in Siemens MRI (1.5T MAGNETOM Sola with the MAGNETOM RT Pro Edition; Siemens Healthineers, Erlangen, Germany), where contrast agent filling is delayed for more than 5 seconds. The area of all slices was measured and converted into volume (ViewRex; TechHeim, Seoul, Korea). Based on these measurements, changes in TTP delay area volume before surgery and 1 week after surgery were calculated (shown in perfusion MRI before and after surgery); improvement of perfusion defect and clinical condition were compared ( Fig. 1), and recanalization of the previously occluded vessel and maintenance of the flow of the donor vessel was confirmed by DSA at 6 months after the surgery. The patients’ neurologic improvement was judged with the interval of initial and postoperative 33-month mRS, defining the patients with improving mRS 2 or more as the improved mRS group, and the patient with no change in mRS or improved less than 1 as the nonimproved mRS group.
Inclusion criteria
Tissue plasminogen activator (tPA) and mechanical thrombectomy were performed according to treatment indications which included patients with NIHSS of 5 or higher due to acute infarction with large-cerebral artery occlusion among adult patients 20-85 years of age who were able to live independently with mRS of 0 or 1 before onset. If the patients failed mechanical thrombectomy or did not receive mechanical thrombectomy because disagreed with the inclusion criteria of mechanical thrombectomy and NIHSS decreased by more than 2 during hospitalization, small infarction volume less than 30 mL, and diffusion perfusion mismatching with TTP delay volume of 60 mL or more, emergency STA-MCA bypass surgery was performed.
Operation method
After harvesting the patient’s parietal or frontal branch of the STA, a craniotomy flap approximately 3×3 cm in size was made along the Sylvian fissure. After opening the dura, several candidate recipient vessels among the M4 branches around the Sylvian fissure were exposed, and the thickest vessels with the best flow were selected as recipients for STA-MCA anastomosis. After anastomosis, if blood flow through the STA measured with the chabel flow meter (Charbel Micro-Flow-probe; Transonics Systems, Inc., Ithaca, NY, USA) was 15 or more, the operation was terminated. If blood flow was low, the frontal branch was used for the bypass additionally. In M1 occlusion, the frontal area was selected as the recipient; in ICA occlusion, the recipient was selected as the vessel with the best flow among the frontal or temporal areas and the greatest thickness ( Fig. 2).
Radiologic evaluation
After the large-vessel occlusion was examined with computed tomography angiography (CTA) before surgery, diffusion-perfusion MRI was performed. The infarction due to the large vessel occlusion was confirmed with DSA, and the treatment direction was determined. The infarctions were classified into four categories based on the site of occurrence : perforator territory, cortical territory, watershed only, or mixed type. Perfusion MRI was checked 1 week after surgery to evaluate improvement in perfusion defect compared with that before surgery. DSA was performed 6 months after surgery to confirm STA patency and recanalization of previously occluded vessels.
Statistical methods
The characteristics of patients were described by frequency count with median with interquartile range. When we compared the variables between patients who showed improved mRS after STA-MCA bypass and those who did not, the Mann-Whitney U test was used in continuous variables, and the chi-squared test or Fisher’s exact test was used in categorized variables. All p-values were two-sided, and a p-value under 0.05 was considered significant. We used R (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria) for all statistical analyses.
RESULTS
Baseline characteristics
The median age of patients that underwent surgery was 63 years, 67.3% were males, and 32.7% were females. The infarction volume was a median 10 mL with the preoperative hypoperfusion area volume a median 101 mL. The NIHSS was median 8. The patient’s last normal to operation time was median 3640 minutes, which was an average of 60.7 hours ( Table 1). Infarction progression before surgery during hospitalization occurred in 26 patients, and their infarction volume increased from 3 mL to 11.3 mL. Their NIHSS was increased on average by 6 points. ICA occlusion patients accounted for the largest proportion (53.3%), followed by M1 occlusion (41.1%) and M2 occlusion (5.6%). tPA was applied in 11 patients, and 14 patients were undergone mechanical thrombectomy but failed and got STA-MCA anastomosis.
Single-barrel bypass was performed on 91 patients and double-barrel bypass was done on 16 patients. Intracranial vessel doppler and indocyanine green angiography were used to confirm bypass patency in all patients during surgery, and all STAs were observed in CTA 6 hours after surgery.
Postoperative characteristics and angiographic findings
Among the 107 patients, perfusion imaging 1 week after surgery was performed in 95 patients; TTP delay completely recovered in 77 patients, partially improved in 10 patients, and localized improvement around the bypass site with global deterioration was seen in eight patients. Based on the analysis of all patients, the median overall hypoperfusion area volume was 0 mL, and the NIHSS score was 3 at the 1-month follow-up, which was improved from the preoperative score of 8. The mRS improved from 3 at discharge to that of 1 at 6 months after discharge. In two cases, burr hole trephination was done because of postoperative chronic subdural hematoma. These patients were discharged without complications after the operation, and no other complications associated with surgery were observed ( Table 2). A total of 68 patients were eligible for follow-up angiography 6 months after discharge. Among the 68 patients, 18 cases of spontaneous recanalization of the occluded vessel were confirmed, and STA occlusion occurred in two cases (2.94% of the total) ( Table 3).
Factors associated with the postoperative outcome
Preoperative NIHSS score and initial mRS were not significantly different between the two groups divided according to the degree of neurologic improvement ( p=0.272, p=0.941). Also, occlusion vessels did not affect the postoperative outcome ( p=0.355). Regarding last normal to operation time, a significant difference was not observed between the two groups ( p=0.391); however, by infarction type, the ratio of mixed type was high in the non-improved mRS group with marginal significance ( p=0.051). In the case of infarction volume, a significant difference was observed in the mRS improved group as 7 and 13.5 mL in the non-improved mRS group ( p=0.010). The preoperative hypoperfusion area median volume in the non-improved mRS group was 82.6 mL and in the improved mRS group was 104.5 mL ( p=0.192). A significant difference was not observed in mismatch volume (defined as hypoperfusion area-infarction volume) between the improved mRS and non-improved mRS groups, either (67.8 and 91 mL, respectively; p=0.416) ( Table 4).
DISCUSSION
Since the discovery of the ischemic core and penumbra in 1977, efforts to preserve the penumbra have been continued [ 2]. Ischemic penumbra is defined as “incomplete infarction” which can be preserved with the recovery of CBF but may fail to recover with continuously low CBF [ 3]. Following the DAWN (DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo) trial and DEFUSE 3 (The Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) trial in 2018, the time limitation of mechanical thrombectomy has been extended to 24 hours from the last normal time. In both trials, when there is a difference between the ischemic core and the penumbra with small infarction, salvage treatment for the penumbra is effective. Which were defined as diffusion-perfusion mismatch calculated by an automated program or diffusion-neurologic status mismatch according to acute infarction volume [ 1, 15]. Studies are evaluating the effects of mechanical thrombectomy on patients within 48 hours from the last normal time [ 5]. But there are no standard measurements for evaluation of salvageable brain tissue and 48 hours from the last normal time is not accepted as standard generally yet. If there is a clear diagnostic method that can identify penumbra, and if the mismatch between the ischemic core and penumbra can be identified, salvage treatment for the brain would be improved. Still, there are patients with large vessel occlusion, who cannot undergo mechanical thrombectomy because of late hospital visit time or low NIHSS with a large hypoperfusion areas. In 1983, Schmiedek et al. [ 18] analyzed extracranial-intracranial (EC-IC) bypass performed at the hyperacute stage, and the result was unsatisfactory. However, perfusion status was not considered and the patient group requiring augmented surgery was not accurately specified. Patients with large infarction volume have extensive brain damage; however, such patients were included in their study and might have affected the surgery outcome adversely. If the inclusion criteria are modified for symptomatic-radiologic mismatch or acute infarction due to diffusion-perfusion mismatch caused by severe perfusion defects, different results can be expected. The EC-IC bypass study and COSS (Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia : the Carotid Occlusion Surgery Study randomized trial) study were two large, prospective studies conducted in 1985 and 2011, respectively, to determine whether EC-IC anastomosis can prevent the recurrence of ischemia in chronic occlusion patients [ 6, 17]. These studies were performed to determine whether surgery is effective in preventing secondary stroke in patients with chronic occlusion. However, patients with acute cerebral infarction and those with severe brain injury during acute cerebral infarction were not included. Furthermore, the results showed that surgery was not beneficial in preventing stroke recurrence. However, in our study, the effects of surgical treatment on preventing the progression of cerebral infarction in patients with acute cerebral infarction caused by large-cerebral vessel occlusion were investigated using perfusion MRI images. Previous studies by Nussbaum et al. [ 16] in 2010, Lee et al. [ 11] in 2013, and Kim et al. [ 10] in 2021 were similar to our study. Based on the analysis of 13, 20, and 18 cases, respectively, the early bypass results were analyzed, and significantly positive results were achieved compared with the previous analyses. However, Lee et al. [ 11] analyzed only patients with recurrence of symptoms during follow-up or infarction recurrence during medication treatment in terms of surgical management. The analysis by Nussbaum et al. [ 16] had several limitations including no record of time between the manifestation of obvious symptoms and surgical treatment. Kim et al. [ 10] analyzed a prospective study of acute infarction patients with urgent STA-MCA anastomosis that is most similar to the present study; however, the number of enrolled patients was small ( Table 5). Patients in our study who underwent emergency STA-MCA anastomosis surgery were subjects with acute ischemic stroke and large vessel occlusion and evaluated with follow-up neurologic status and perfusion MRI. The median last normal to operation time for all patients was 60.6 hours, and 57 patients were within 72 hours of the last normal to operation time. Unlike high flow bypass, there was no statistical difference between patients who underwent bypass surgery within 3 days and those who underwent bypass surgery over 3 days ( p=0.3054). Although a therapeutic time window for ischemic penumbra of less than 1 day was reported in many studies, there are few studies about penumbra existence for more than a few days under specific circumstances like an inflammatory process [ 14]. The spontaneous recanalization rate of 26.5% indicates the possibility of an acute occlusion lesion being misidentified as chronic occlusion. However, based on the finding that the patency of the STA is well maintained, even in the case of recanalization, it is possible that STA-MCA bypass can support survival of the hypoperfusion area. Maintaining the collateral using bypass in the acute stage aids in spontaneous recanalization and acts as a tissue saver until the blood vessel opens. In addition, decreased cerebrovascular reactivity and deficits of white matter caused by occlusion vessels could be recovered by STA-MCA anastomosis [ 13]. With large ischemic penumbra and eagerness for blood flow with high demand, even low flow bypass can be effective for patency and easily spreading blood flow. The preoperative factor that showed a significant difference in improving mRS score was infarction volume. Preop NIHSS, initial mRS, or infarction location did not affect the improvement of mRS. The hypoperfusion volume and mismatch volume which can be defined as ischemic penumbra analyzed based on the difference between hypoperfusion volume and infarction volume showed no statistical difference in improving mRS ( Table 5). Jo et al. [ 8] reported an interesting study about the effectiveness of EC-IC bypass analysis by radiologic results in 2022. The authors analyzed radiological improvement of CT perfusion before and after EC-IC bypass. Even though there is a difference between our study about the improvement of mismatch volume because our study analyzed according to patients’ status and this study analyzed under radiologic findings, this study can be the important discovery of EC-IC bypass effect on hypoperfusion status. This is an interesting finding of our study. Under clear guidelines for STA-MCA anastomosis, small infarction volume could be more important than large hypoperfusion volume for patients’ neurological improvement. We think there is a possibility that a volume difference might appear if the analysis is performed with a larger dataset, but this can be one clue that simple calculation of ischemic penumbra according to hypoperfusion area and infarction area is hard. Regarding this finding, we have to be in agony for evaluating the ischemic core and ischemic penumbra precisely.
In our study, hypoperfusion area volume was calculated using a TTP image of perfusion MRI. Flow restoration, such as bypass in hemodynamic failure stage II, was proven to help improve hemodynamics [ 7]. However, the time-consuming positron emission tomography scans performed on patients in the acute stage might not be suitable. Originally, the ischemic penumbra is defined as a failure of the cortical evoked potential with decreased CBF [ 3]. Maps for circulation time are suggested as methods for confirming the penumbra area in CT or MR perfusion images; however, clear parameters have not been identified [ 4, 20]. And several studies used multiple images for evaluating ischemic penumbra but not the one that is superior to others [ 12]. Kim et al. [ 9] investigated perfusion MRI can be a parameter for early progression of acute infarction. DEFUSE 3 trial used automated software (RAPID; RapidAI, Menlo Park, CA, USA) for defining diffusion-perfusion mismatching [ 1]. In our analysis, surgical treatment was performed using more intuitive and visualized TTP as reference data, where contrast agent filling is delayed for more than 5 seconds. And previously decreased hypoperfusion area was shown dramatic improvement after surgery. Which is more cost-effective than other software programs and intuitive to easily used. CBF and cerebral blood volume maps vary considerably, limiting constant intuitive evaluation of the degree of perfusion defects. TTP images provide information immediately and are useful to easily evaluate the degree of improvement in perfusion ( Fig. 1). In this study, the median last normal to operation time was 60.6 hours when making decisions based on TTP images, which helps in mediating the determination of surgical management.
Limitation
We do not perform three phase CTA so we don’t have any data in these retrospective series. Even though we determined perfusion MRI is a good tool for evaluating collateral flow, there could be few differences between three phase CT angiography and perfusion MRI.
There are a few patients who had a longer time interval between the last normal time and operation time, and there could be a limitation in interpretation. But the authors expect these limitations could be overcome under larger data.
CONCLUSION
Improvement of perfusion defect within a short time by low f low bypass like STA-MCA anastomosis was identified through perfusion MRI and there was no more ischemic injury after anastomosis. In particular, patients with small infarction volume can achieve a much better outcome. Time is important when actively managing patients with large-vessel occlusion, and ischemic penumbra can be detected easily with TTP images of perfusion MRI.
Fig. 1.
Perfusion magnetic resonance imaging images of pre-operation and post-operation. A : cerebral blood flow (CBF) image of pre-operation. B : cerebral blood volume (CBV) image of pre-operation. C : Time-to-peak (TTP) image of pre-operation. D : CBF image at postoperative day 7. E : CBV image at postoperative day 7. F : TTP image at postoperative day 7. 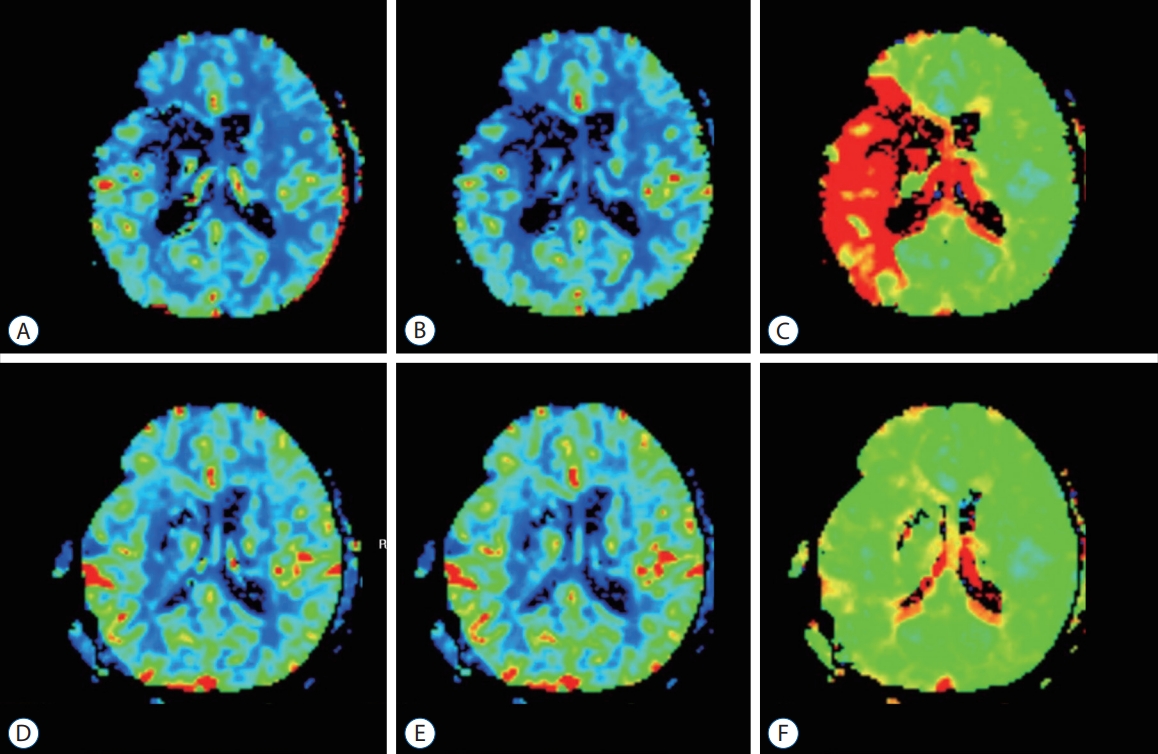
Fig. 2.
Magnified surface image of postoperative computed tomography angiography of superficial temporal artery to middle cerebral artery anastomosis. 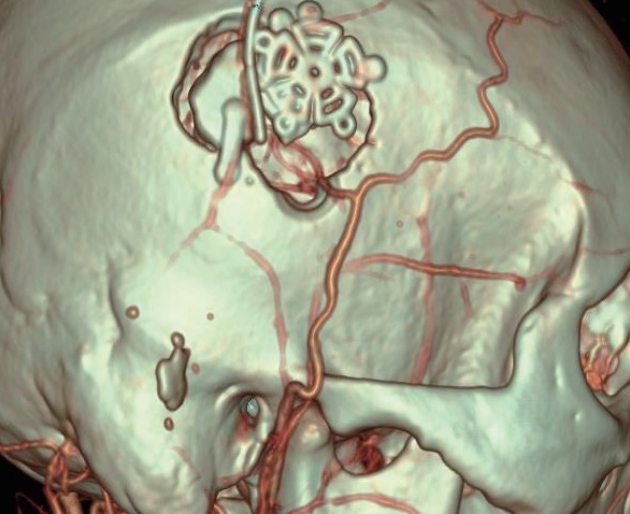
Table 1.
|
Emergency STA-MCA bypass (n=107) |
|
Age (years) |
63 (55-73) |
|
Sex |
|
|
Female |
35 (32.7) |
|
Male |
72 (67.3) |
|
Infarction volume |
10 (5-20) |
|
Preoperative perfusion defect volume |
101 (62.3-178.4) |
|
Preoperative NIHSS score |
8 (4-13) |
|
Last normal to operation time (minutes) |
3,640 (1,790-10,217.5) |
|
Occlusion site |
|
|
ICA |
57 (53.3) |
|
M1 |
44 (41.1) |
|
M2 |
6 (5.6) |
|
Bypass technique |
|
|
Single barrel |
91 (85.0) |
|
Double barrel |
16 (15.0) |
Table 2.
Postoperative characteristics of patients
|
Emergency STA-MCA bypass (n=107) |
|
Postoperative perfusion defect volume (mL) |
0 (0-0) |
|
1 month follow up NIHSS score |
3 (1-6) |
|
mRS score |
|
|
Discharge |
3 (1-4) |
|
6 months |
1 (0-3) |
|
Complication |
2 (0.02) |
Table 3.
Findings of postoperative 6 months follow up digital subtraction angiography
|
6-month follow-up DSA (n=68) |
|
Recanalization of the occluded vessel |
18 (26.5) |
|
STA patency |
|
|
Patent |
66 (97.1) |
|
Occluded |
2 (2.94) |
Table 4.
Factors associated with the postoperative outcome
|
Improved mRS (n=43) |
Non-improved mRS (n=56) |
p-value*
|
|
Preoperative NIHSS score |
7 (4-11) |
8 (3-13) |
0.272 |
|
Initial mRS score |
3 (3-4) |
4 (2-4) |
0.941 |
|
Infarction volume (mL) |
7.0 (5.0-12.2) |
13.5 (6.0-23.0) |
0.010 |
|
Preoperative hypoperfusion volume (mL) |
82.6 (63-119.2) |
104.5 (59.1-186.5) |
0.192 |
|
Mismatch volume, hypoperfusion−infarction volume (mL) |
67.8 (48.2-107.5) |
91.0 (47.3-158.0) |
0.416 |
|
Occlusion vessel |
|
|
0.355 |
|
ICA |
22 (51.2) |
29 (51.8) |
|
|
M1 |
20 (46.5) |
22 (39.3) |
|
|
M2 |
1 (2.3) |
5 (8.9) |
|
|
Last normal to operation time (minutes) |
3266 (1958-7139) |
4089 (1704-14631) |
0.391 |
|
Infarction type |
|
|
0.051 |
|
Perforator |
12 (27.9) |
8 (14.3) |
|
|
Cortical |
13 (30.2) |
15 (26.8) |
|
|
Watershed |
7 (16.3) |
4 (7.1) |
|
|
Mixed |
10 (23.3) |
26 (46.4) |
|
|
N/A |
1 |
3 |
|
Table 5.
The outcome of other studies about ec-Ic bypass for large vessel occlusion
|
Schmiedek et al. [18] |
EC-IC bypass study group [6] |
Coss [17] |
Nussbaum et al. [16] |
Lee et al. [11] |
Kim et al. [10] |
|
Published |
1983 |
1985 |
2011 |
2010 |
2013 |
2021 |
|
Study design |
Retrospective |
Prospective |
Prospective |
Retrospective |
Retrospective |
Prospective |
|
Inclusion criteria |
Young patients, focal neurologic deficit, negative initial CT finding |
Not mentioned |
ICA occlusion with TIA or ischemic stroke within 120 days |
Not mentioned |
After recurrence or aggravation |
Acute infarction |
|
Patient number |
Surgical only, 11 |
Medical, 714 |
Surgical, 97 |
Surgical only, 13 |
Surgical only, 20 |
Surgical only, 18 |
|
Surgical, 663 |
Medical, 98 |
|
Vessel |
ICA : 5 |
ICA occlusion with recurrent symptoms |
Symptomatic ICA occlusion |
ICA : 10 |
ICA : 40% |
ICA : 55.6% |
|
MCA : 6 |
Medical : 20.6% |
M1 : 3 |
MCA : 60% |
MCA : 44.4% |
|
Surgical : 21.1% |
|
Interval |
7-11 hours, 24 hours in one case |
|
Within 120 days |
Not mentioned |
Within 7 days |
2.61±2.21 days |
|
Outcome |
Improvement : 6 |
No benefit of surgery for stroke recurrence |
No benefit of surgery for stroke recurrence |
No further stroke |
Good : 17 |
Improvement : 75% |
|
Died in early stage : 3 |
progression : 11 |
Fair : 2 |
|
Improvement : 2 |
Poor/died : 1 |
References
1. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al : Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 378 : 708-718, 2018    2. Astrup J, Symon L, Branston NM, Lassen NA : Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke 8 : 51-57, 1977   3. Back T : Pathophysiology of the ischemic penumbra--revision of a concept. Cell Mol Neurobiol 18 : 621-638, 1998    4. Demeestere J, Wouters A, Christensen S, Lemmens R, Lansberg MG : Review of perfusion imaging in acute ischemic stroke: from time to tissue. Stroke 51 : 1017-1024, 2020   5. Desai SM, Haussen DC, Aghaebrahim A, Al-Bayati AR, Santos R, Nogueira RG, et al : Thrombectomy 24 hours after stroke: beyond DAWN. J Neurointerv Surg 10 : 1039-1042, 2018   6. EC/IC Bypass Study Group : Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med 313 : 1191-1200, 1985   7. Grubb RL Jr, Powers WJ, Clarke WR, Videen TO, Adams HP Jr, Derdeyn CP, et al : Surgical results of the carotid occlusion surgery study. J Neurosurg 118 : 25-33, 2013    9. Kim H, Kim Y, Kim YW, Kim SR, Yang SH : Perfusion-weighted MRI parameters for prediction of early progressive infarction in middle cerebral artery occlusion. J Korean Neurosurg Soc 59 : 346-351, 2016    10. Kim JH, Yoon W, Kim CK, Roh H, Bae HJ, Kwon TH, et al : Efficacy and safety of timely urgent superficial temporal artery-to-middle cerebral artery bypass surgery in patients with acute ischemic stroke: a single-institutional prospective study and a pooled analysis. Cerebrovasc Dis 50 : 34-45, 2021    11. Lee SB, Huh PW, Kim DS, Yoo DS, Lee TG, Cho KS : Early superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke. Clin Neurol Neurosurg 115 : 1238-1244, 2013   13. McKetton L, Venkatraghavan L, Rosen C, Mandell DM, Sam K, Sobczyk O, et al : Improved white matter cerebrovascular reactivity after revascularization in patients with steno-occlusive disease. AJNR Am J Neuroradiol 40 : 45-50, 2019    14. Moustafa RR, Baron JC : Pathophysiology of ischaemic stroke: insights from imaging, and implications for therapy and drug discovery. Br J Pharmacol 153 Suppl 1( Suppl 1):S44-S54, 2008     15. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al : Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 378 : 11-21, 2018  16. Nussbaum ES, Janjua TM, Defillo A, Lowary JL, Nussbaum LA : Emergency extracranial-intracranial bypass surgery for acute ischemic stroke. J Neurosurg 112 : 666-673, 2010   17. Powers WJ, Clarke WR, Grubb RL Jr, Videen TO, Adams HP Jr, Derdeyn CP : Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA 306 : 1983-1992, 2011    18. Schmiedek P, Olteanu-Nerbe V, Marguth F : Timing of extracranial-intracranial arterial bypass surgery with special reference to acute cerebral ischaemia. Neurosurg Rev 6 : 19-24, 1983    19. Tsivgoulis G, Safouris A, Katsanos AH, Arthur AS, Alexandrov AV : Mechanical thrombectomy for emergent large vessel occlusion: a critical appraisal of recent randomized controlled clinical trials. Brain Behav 6 : e004182016    20. Vilela P, Rowley HA : Brain ischemia: CT and MRI techniques in acute ischemic stroke. Eur J Radiol 96 : 162-172, 2017  
|
|


















