Sani, Kurniawan, Hamdan, and Swatan: Delayed Cerebral Ischemia after Embolization in Ruptured Spinal Arteriovenous Fistula with Subarachnoid Hemorrhage : A Case Report
Abstract
Delayed cerebral ischemia (DCI) remains a devastating complication in subarachnoid hemorrhage (SAH), however, there were no present reports that is associated with a ruptured spinal arteriovenous fistula (sAVF). We would like to present a rare case of DCI following embolization of a ruptured perimedullary sAVF. Initially, the patient clinical symptoms mimic a SAH caused by a ruptured intracranial aneurysm. Further evaluation revealed that the SAH was caused by a ruptured perimedullary sAVF and the patient’s condition improved following the embolization procedure. Three days later, the patient developed an acute left-sided facial and motor weakness, which persisted until the patient was discharged on the day-15 onset. A magnetic resonance imaging and angiography is performed 1.5 years after discharge and revealed no signs of cerebral infarction and hemorrhage. In this paper, we reported DCI after embolization in a ruptured sAVF with SAH, supported by evidence from the current literature. We would like to also stress the importance of complete spinal and cerebral vessel imaging to reveal the underlying abnormalities and determine the most appropriate intervention.
Key Words: Cerebral infarction · Therapeutic embolization · Subarachnoid hemorrhage · Arteriovenous fistula, spine.
INTRODUCTION
Delayed cerebral ischemia (DCI) is one of the most devastating complications in subarachnoid hemorrhage (SAH) which may be an indicator of poor outcome [ 5]. Although the exact mechanism of DCI is largely unknown, several studies and case reports have highlighted a link between SAH and DCI due to cerebral vasospasm [ 4]. The majority of SAH is caused by rupture of intracranial aneurysm, however, there is a small percentage of SAH associated with spinal vascular malformation [ 9, 15]. From the best of our knowledge, there is only one case report that correlates spinal arteriovenous fistula (sAVF) rupture with SAH [ 15], but there are none that directly link ruptured sAVF with DCI. Therefore, we would like to present a rare case of DCI following embolization of a ruptured perimedullary sAVF.
CASE REPORT
A 51-year-old male presented to the emergency room with an abrupt severe headache (numerical rating scale of 10) and vomiting. The patient’s history was significant for alcohol use and excessive smoking. The patient was previously known well with no significant past medical history and currently not consuming anticoagulants. Family history of stroke, cancer, and other vascular malformation was denied.
The patient blood pressure upon admission was 150/90 mmHg. Neurological examination revealed a positive nuchal rigidity. We found no other abnormalities during the initial examination. The patient’s Glascow coma scale (GCS) was 15, mental status was normal, no cranial nerves deficit was found, motor and sensory examination of all four limbs were within normal limits, and the bladder was empty.
A head non-contrast computed tomography conducted upon arrival revealed an acute SAH on the left subarachnoid space in front of the pontomedullary junction ( Fig. 1). The modified Fischer scale was 1 and the Hunt and Hess scale was 2. The diagnosis of SAH was confirmed and oral nimodipine therapy was initiated with a dosage of 60 mg every 4 hours. A cerebral digital subtraction angiography (DSA) was arranged 3 days after admission and revealed a perimedullary sAVF with an arterial feeder from anterior spinal artery and draining vein to the superficial perimedullary vein ( Fig. 2). The microcatheter (Magic 1.2F Flow-Dependent Microcatheter; Balt, Montmorency, France) was inserted to the feeding artery and embolization procedure was performed using Nbuytl-2-cyanoacrylate (NBCA) (Histoacryl ®; B-Braun, Melsungen, Germany) under general anesthesia. Complete obliteration of the sAVF was confirmed using cerebral DSA. The patient condition improved with no neurological deficit or sequel within 24 hours after procedure. Three days following the procedure (day-6 onset), the patient complained of an acute onset of left-sided facial and limb weakness. The patient’s GCS was 15 with a blood pressure of 150/90 mmHg. Neurological examination was significant for nuchal rigidity, left central-type facial and lingual palsy, and left-sided hemiparesis with a Medical Research Council (MRC) score of 4- for the upper extremities and 3 for the lower extremities. The diagnosis of DCI was clinically established and we continued the administration of nimodipine until the patient’s condition stabilized. The patient was discharged on day-15 onset with a modified Rankin Scale of 4 and a slight improvement on the left-sided hemiparesis (upper extremities MRC 4, lower extremities MRC 3). Follow-up at the neurological clinic was arranged in 1 week after discharge, but the patient never came to the clinic and considered loss to follow up.
After 1.5 years, the patient was incidentally referred back to our outpatient clinic for further evaluation on neuroimaging. He had no complaints of headache/vomiting, the left-sided facial and lingual palsy were absent, and the left-sided hemiparesis improved (upper extremities MRC 5, lower extremities MRC 4+). A head magnetic resonance imaging (MRI) and magnetic resonance angiography examination revealed a complete obliteration of the sAVF and no signs of cerebral infarction or hemorrhage ( Fig. 3).
DISCUSSION
The challenges in diagnosing spinal vascular malformation A spinal vascular malformation is a rare disorder, comprising less than 10% of intracranial and spinal vascular malformations [ 8]. The clinical course of sAVF is mostly benign and symptoms resulting from venous congestion and hypoxia may be vague. This is why most of the patients with sAVF—at the time of diagnosis—already have considerable neurological deficits, including gait difficulty, micturition or defecation disturbances, and even upper motor neuron or lower motor neuron involvements [ 7]. The patient in our case presented with hallmark symptoms of intracranial SAH. There are evidences from previous studies where in a rare occasion, blood from the ruptured sAVF may reflux toward the cerebral circulatory, hence, a cranial SAH occurs [ 15]. Without extensive cerebral vasculature imaging, clinicians may unable to localize the cause of SAH, especially in the case of spinal vascular malformations. In 2016, Takai [ 12] introduced a classification system that classifies spinal arteriovenous shunts based on the sites and types of the lesion. It was classified into five groups, namely : 1) dural AVF, 2) intradural intramedullary glomus arteriovenous malformation (AVM), 3) intradural intramedullary juvenile AVM, 4) perimedullary AVF, and 5) extradural AVF. Perimedullary AVF was further classified into three subtypes based on the feeding artery and AVF size : single feeder and small AVF as type IVa; multiple feeders and medium AVF as type IVb; multiple feeders and giant AVF as type IVc. While extradural AVF was further classified into two : with intradural venous drainage for type Va, and without intradural venous drainage for type Vb. Based on this description, our case could be categorized under type IVa with a single artery feeder and small AVF size.
DCI caused by SAH, what do we know up to now?
DCI is defined as “The occurrence of focal neurological impairment … or a decrease of at least 2 points on the Glasgow Coma Scale … This should last for at least 1 hour, is not apparent immediately after aneurysm occlusion, and cannot be attributed to other causes by means of clinical assessment, CT or MRI scanning of the brain, and appropriate laboratory studies” [ 14]. The occurrence of DCI in SAH is highly unpredictable because no present studies have been conducted to analyze the risk factors of developing a DCI. In addition, there are no approved treatment for DCI due to its complex and multifactorial nature, leading to a poor outcome for the patient [ 3]. Currently, the exact mechanism of DCI remains poorly understood. Several theories are proposed to explain DCI in SAH as follows : 1) vascular dysfunction, which encompasses cerebral vasospasm, microcirculatory dysfunction, formation of microthrombus and thrombo-inflammation, and entrance of blood in subarachnoid space to the brain parenchyma through glymphatic and meningeal lymphatic pathway; 2) inflammatory reaction either from systemic inflammation following a SAH or through cell-mediated responses such as glial cells and leukocytes; and 3) spreading depolarization following a SAH due to cellular hyperemic response and increased metabolic demand caused by disruption in ion homeostasis [ 1, 2]. In this case, we assume that the DCI was caused by microthrombus formation and thrombo-inflammation process. This theory may be supported by the patient’s heavy smoking habit and absence of lesion in the brain MRI evaluation. However, the exact cause of this DCI remains unclear since we were unable to confirm nor exclude the other hypothesis. In our case, the diagnosis of DCI was established due to the patient’s clinical presentation of sudden onset hemiparesis, persisting more than an hour, and, occurring in the 6th day of admission. This finding is consistent with reports from previous studies where DCI often observed during the 4-10th day after the onset of SAH [ 11]. Although the embolization procedure conducted carry a risk of developing cerebral ischemia [ 10], we would like to argue that the interventional procedure received by the patient did not contribute to the DCI. This is be cause during the first 24 hours after the procedure, the patient’s condition improved with no neurological deficits.
Treatment options and patient outcome
To this date, there is limited evidence regarding treatment options in sAVF. Although treatment preferences are mainly determined by the type of sAVF, both surgical intervention and endovascular therapy remain the most common options. Previous studies reported no significant difference in clinical outcome and rate of complications in patients receiving the surgical intervention and endovascular therapy. However, endovascular therapy remained the first-line treatment choice for having less intraprocedural risk compared to the surgical intervention. In this case, we favor the embolization procedure using NBCA because it has a better safety profile with the same efficacy as surgical procedure [ 6]. For the DCI, nimodipine is the recommended treatment option due to its beneficial effect in preventing vasospasm—a common culprit of DCI caused by SAH [ 3, 4]. Several alternative treatment options include induced hypertension, volume optimization, endovascular approach, increasing cardiac output, and hemoglobin optimization [ 3]. The patient’s condition improved significantly compared to the time of initial presentation. After 1.5 years of follow-up, the only neurological sequelae persisted in the patient is a hemiplegic gait of the left leg. This finding is consistent with a systematic review that reports better clinical course for DCI patients with no lesions in MRI examination [ 13].
CONCLUSION
DCI remained a devastating complication of SAH, even though it was caused by a non-aneurysmal etiology, located in spinal vasculatures, and with a small bleeding volume. A complete examination of the spinal and cerebral vessels is needed to reveal the underlying abnormalities and determine the most appropriate intervention.
Fig. 1.
An axial non-contrast head computed tomography-scan revealed subarachnoid hemorrhage in the left pontomedullary junction (arrow). 
Fig. 2.
A cerebral digital subtraction angiography in anteroposterior and oblique view following injection from left vertebral artery revealed a spinal arteriovenous fistula (sAVF) (red circle) with feeding artery from anterior spinal artery (yellow arrow) and drains to perimedullary vein (orange arrow) revealed the sAVF before embolization (A and B), during embolization procedure using N-buytl-2-cyanoacrylate (Histoacryl®; B-Braun, Melsungen, Germany) in a single super-selective injection (white arrow, microcatheter tip) of the arterial feeder (C and D), and complete obliteration after embolization procedure (E and F). 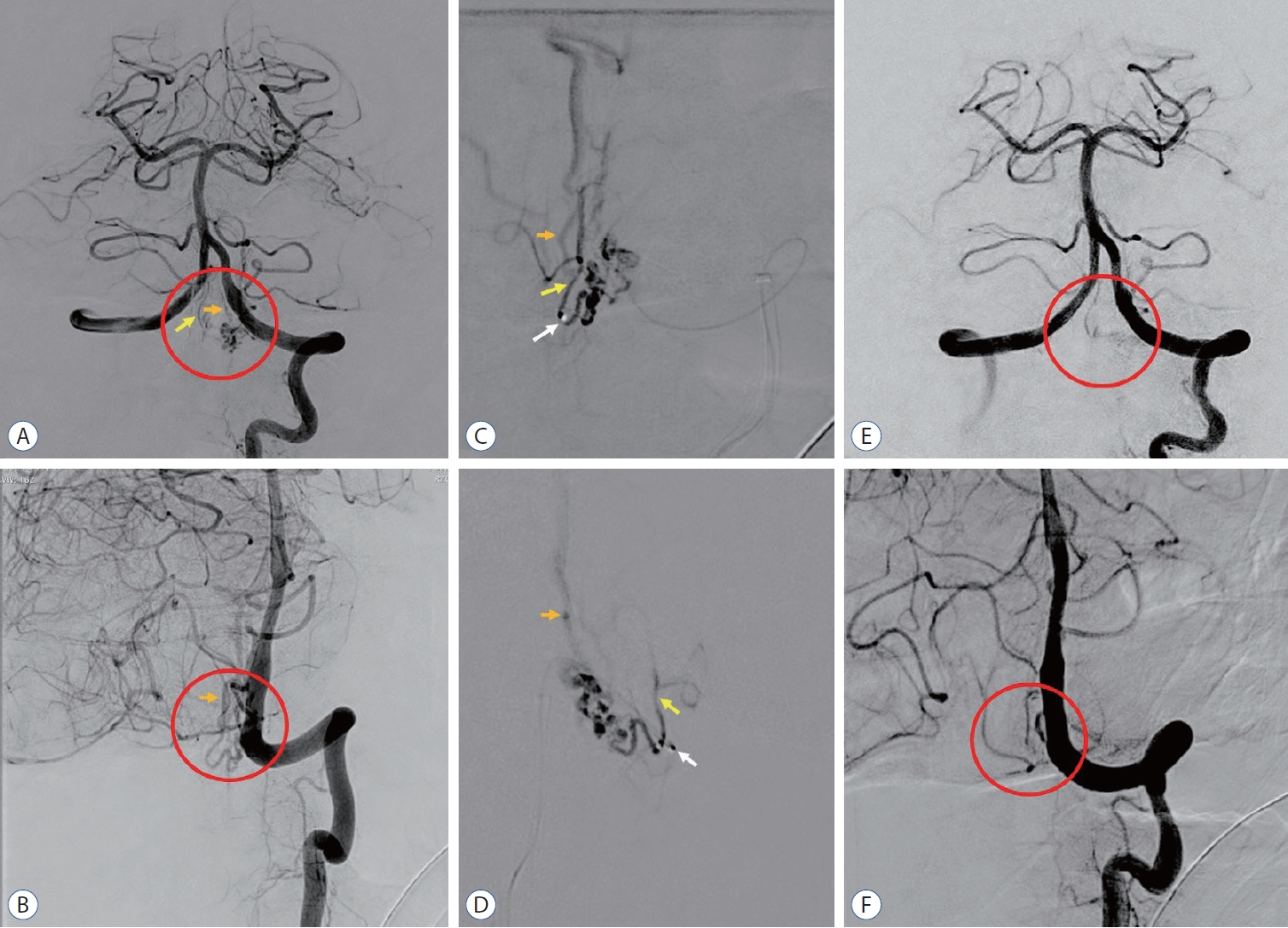
Fig. 3.
Axial fluid-attenuated inversion recovery-sequence (A-C), coronal (D), and sagittal T2 (E) sequence of brain magnetic resonance imaging with contrast; and magnetic resonance angiography of cerebral blood vessels (F) conducted 1.5 years after the onset of delayed cerebral ischemia revealed no lesions or vascular abnormalities. 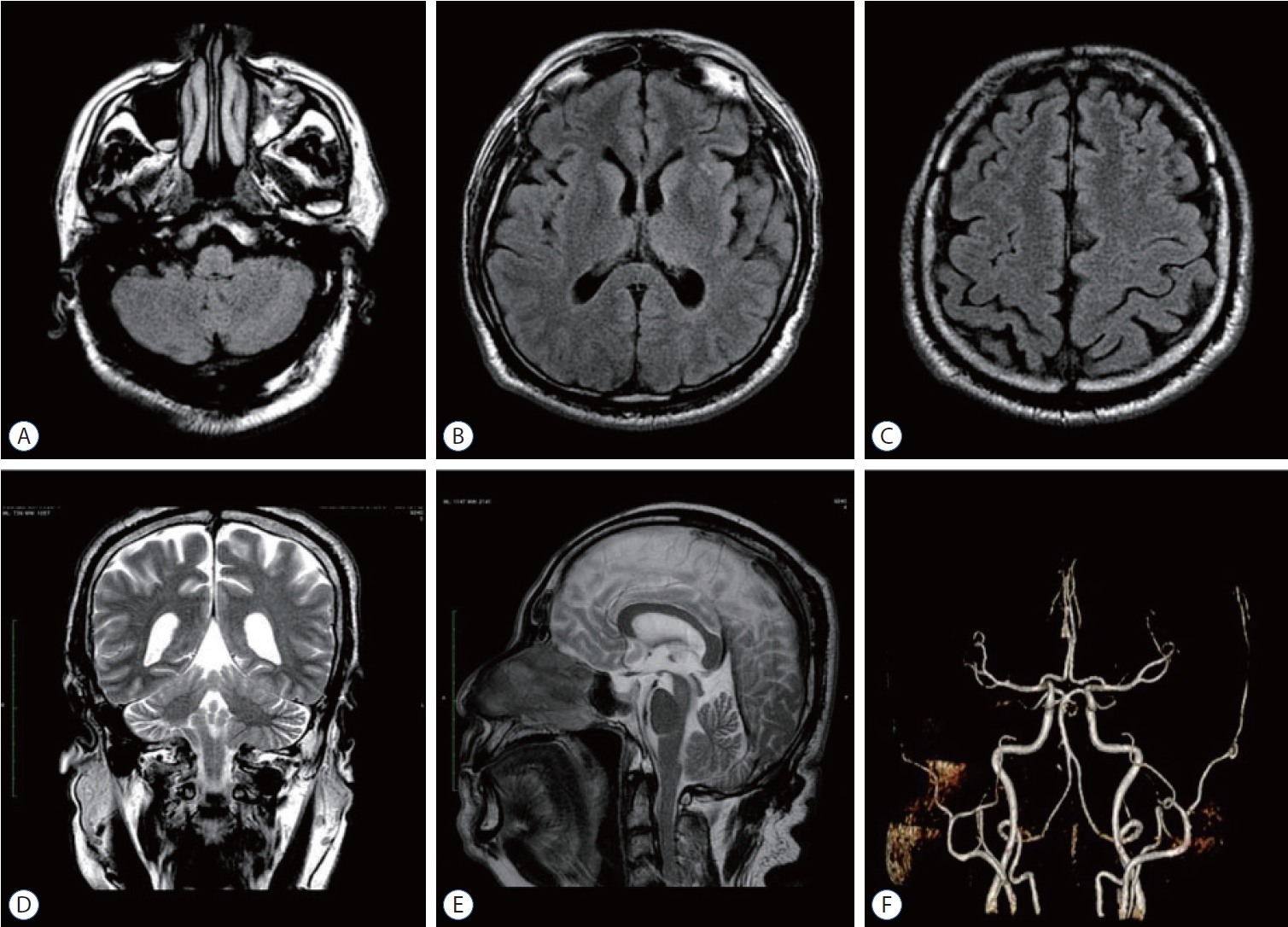
References
1. Ayata C, Lauritzen M : Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol Rev 95 : 953-993, 2015    2. Dodd WS, Laurent D, Dumont AS, Hasan DM, Jabbour PM, Starke RM, et al : Pathophysiology of delayed cerebral ischemia after subarachnoid hemorrhage: a review. J Am Heart Assoc 10 : e021845, 2021    3. Francoeur CL, Mayer SA : Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care 20 : 277, 2016    4. Goursaud S, Martinez de Lizarrondo S, Grolleau F, Chagnot A, Agin V, Maubert E, et al : Delayed cerebral ischemia after subarachnoid hemorrhage: is there a relevant experimental model? A systematic review of preclinical literature. Front Cardiovasc Med 8 : 752769, 2021    5. Ikram A, Javaid MA, Ortega-Gutierrez S, Selim M, Kelangi S, Anwar SMH, et al : Delayed cerebral ischemia after subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 30 : 106064, 2021   6. Koch MJ, Stapleton CJ, Agarwalla PK, Torok C, Shin JH, Coumans JV, et al : Open and endovascular treatment of spinal dural arteriovenous fistulas: a 10-year experience. J Neurosurg Spine 26 : 519-523, 2017   7. Krings T, Geibprasert S : Spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol 30 : 639-648, 2009    8. Murai S, Hiramatsu M, Suzuki E, Ishibashi R, Takai H, Miyazaki Y, et al : Trends in incidence of intracranial and spinal arteriovenous shunts: hospital-based surveillance in Okayama, Japan. Stroke 52 : 1455-1459, 2021   9. Neifert SN, Chapman EK, Martini ML, Shuman WH, Schupper AJ, Oermann EK, et al : Aneurysmal subarachnoid hemorrhage: the last decade. Transl Stroke Res 12 : 428-446, 2021    10. Qureshi AI, Luft AR, Sharma M, Guterman LR, Hopkins LN : Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures: part II--clinical aspects and recommendations. Neurosurgery 46 : 1360-1375, 2000   11. Sarrafzadeh AS, Vajkoczy P, Bijlenga P, Schaller K : Monitoring in neurointensive care - the challenge to detect delayed cerebral ischemia in high-grade aneurysmal SAH. Front Neurol 5 : 134, 2014    12. Takai K : Spinal arteriovenous shunts: angioarchitecture and historical changes in classification. Neurol Med Chir (Tokyo) 57 : 356-365, 2017    13. van der Kleij LA, De Vis JB, Olivot JM, Calviere L, Cognard C, Zuithoff NP, et al : Magnetic resonance imaging and cerebral ischemia after aneurysmal subarachnoid hemorrhage: a systematic review and metaanalysis. Stroke 48 : 239-245, 2017   14. Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, et al : Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 41 : 2391-2395, 2010   15. Zhao J, Esemen Y, Rane N, Nair R : Intracranial subarachnoid haemorrhage caused by cervical spinal dural arteriovenous fistulas: case report. Front Neurol 12 : 685332, 2021   
|
|



















