Lee, Choi, Ha, and Lim: Predictive Factors of First-Pass Effect in Patients Who Underwent Successful Endovascular Thrombectomy for Emergent Large Vessel Occlusion
Abstract
Objective
The primary treatment goal of current endovascular thrombectomy (EVT) for emergent large-vessel occlusion (ELVO) is complete recanalization after a single maneuver, referred to as the ŌĆśfirst-pass effectŌĆÖ (FPE). Hence, we aimed to identify the predictive factors of FPE and assess its effect on clinical outcomes in patients with ELVO of the anterior circulation.
Methods
Among the 129 patients who participated, 110 eligible patients with proximal ELVO (intracranial internal carotid artery and proximal middle cerebral artery) who achieved successful recanalization after EVT were retrospectively reviewed. A comparative analysis between patients who achieved FPE and all others (defined as a non-FPE group) was performed regarding baseline characteristics, clinical variables, and clinical outcomes. Multivariate logistic regression analyses were subsequently conducted for potential predictive factors with p<0.10 in the univariate analysis to determine the independent predictive factors of FPE.
Results
FPE was achieved in 31 of the 110 patients (28.2%). The FPE group had a significantly higher level of functional independence at 90 days than did the non-FPE group (80.6% vs. 50.6%, p=0.002). Pretreatment intravenous thrombolysis (IVT) (odds ratio [OR], 3.179; 95% confidence interval [CI], 1.025-9.861; p=0.045), door-to-puncture (DTP) interval (OR, 0.959; 95% CI, 0.932-0.987; p=0.004), and the use of balloon guiding catheter (BGC) (OR, 3.591; 95% CI, 1.231-10.469; p=0.019) were independent predictive factors of FPE.
Conclusion
In conclusion, pretreatment IVT, use of BGC, and a shorter DTP interval were positively associated with FPE, increasing the chance of acquiring better clinical outcomes.
Key Words: Acute ischemic stroke ┬Ę First-pass effect ┬Ę Reperfusion ┬Ę Thrombectomy.
INTRODUCTION
Endovascular thrombectomy (EVT) is the standard treatment for patients with acute ischemic stroke (AIS) due to emergent large-vessel occlusion (ELVO) of the anterior circulation [ 24]. Efficacy of EVT has been demonstrated in several landmark randomized controlled trials [ 4, 12, 26]. With the gradual development of thrombectomy techniques and equipment, the reperfusion rate has improved to Ōēź80% in patients with ELVO [ 2, 31]. There has been a shift in interest toward a rapid, high level of revascularization that may lead to a favorable prognosis [ 3, 7, 18]. Recent studies have shown that the number of device passes required for recanalization serves as a predictive factor of functional outcome [ 3, 28]. It has also been suggested that patients with a modified Thrombolysis in Cerebral Infarction (mTICI) score of 3 achieved better clinical outcomes as compared with those with a score of mTICI 2b [ 7, 18]. This indicates that complete recanalization after a single maneuver might be the primary treatment goal of the current EVT techniques. Recently, the first-pass effect (FPE) has intrigued neuro-interventionists; it is defined as the achievement of a complete reperfusion of the AIS with a single pass of a device without rescue therapy during EVT, and its significant correlation with favorable outcomes has been well described in the literature [ 35]. It is essential to identify predictive factors of the FPE to improve outcomes of the EVT by preoperatively modifying the relevant factors. Despite consistent reports regarding a significant correlation between FPE and good functional outcomes of EVT [ 8, 16, 23], controversial data exist regarding predictors of FPE in patients undergoing EVT [ 8, 16, 23, 27, 30]. Moreover, the majority of the previously published studies which identify predictors of FPE have compared the FPE group with all others comprising a heterogeneous cohort, including those with mTICI Ōēż2a [ 8, 27, 33]. Hence, in this retrospective study, we aimed to identify predictive factors of FPE and assess its effects on clinical outcomes in a limited number of patients who achieved successful recanalization after EVT for ELVO of the anterior circulation.
MATERIALS AND METHODS
The current study was approved by the Institutional Review Board (IRB) of Korea University Ansan Hospital (IRB No. 2022AS0264). Given that it was designed retrospectively, the written informed consent was waived.
Study design
We retrospectively collected electronic data from the patients with AIS who had been hospitalized at our academic institution between March 2015 and December 2021. Among consecutive patients with AIS due to ELVO of the anterior circulation treated by EVT, those who only had successful recanalization (achieving mTICI 2b/3 on the final cerebral angiography) were included. Additionally, we limited this study to occlusion of the intracranial internal carotid artery (ICA) and proximal middle cerebral artery (MCA-M1) established using cerebral angiography. A flowchart of patient enrollment is demonstrated in Fig. 1.
Patient data and variables
Baseline characteristics of the patients included sex, age, medical history, history of antithrombotic medications, pre-procedural National Institutes of Health Stroke Scale (NIHSS) score assessed by a stroke neurologist at the department of emergency medicine, and etiology of AIS, which was classified based on the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria [ 1]. Pre-procedural variables included the Alberta Stroke Program Early CT Score (ASPECTS) on admission based on brain computed tomography (CT) scan, site of vascular occlusion, clot burden score assessed on baseline brain CT angiography scored on a scale of 0 to 10 [ 25], onset-to-door (OTD) time defined as the time from stroke onset to hospital arrival, and pretreatment intravenous thrombolysis (IVT) with tissue plasminogen activator (tPA). Procedural variables included door-to-puncture (DTP) time defined as the time from hospital arrival to procedure, onset-to-puncture (OTP) time defined as the time to procedure from stroke onset, procedure time, use of balloon guiding catheter (BGC), and first-line EVT strategy. All the time-related variables were assessed in 5-minute intervals.
EVT protocol
All procedures were performed by three experienced neurointerventionists in the neuro-angiography suite under local anesthesia alone or in combination with conscious sedation depending on the patientŌĆÖs condition. Prior to the EVT, the eligible patients were administered tPA intravenously within 4.5 hours of stroke onset at a maximum dose of 0.9 mg/kg by stroke neurologists according to the conventional guidelines [ 24]. EVT was performed using either the second-generation stent retriever (Solitaire, Medtronic, Irvine, CA, USA; Trevo, Stryker, Fremont, CA, USA) or an aspiration catheter (SOFIA, Microvention, Tustin, CA, USA), or both at the attending neurointerventionistsŌĆÖ discretion. An 8-F BGC (Cello, Medtronic; FlowGate2, Stryker) was also used to reduce the embolic burden in the new territory by achieving proximal flow control at the discretion of the attending neuro-interventionists. Otherwise, a 6- or 7-F Shuttle sheath (Cook Medical, Bloomington, IN, USA) was used as the guiding catheter.
In most patients, clot retraction was performed using a stent retriever with an 8-F BGC. Using a microcatheter, a stent retriever was advanced and placed at the occlusion site for 3-5 minutes, followed by inflation of a balloon of BGC. The stent was retrieved with a manual aspiration of the BGC using a 60-mL syringe. In some patients, a coaxial intermediate catheter was additionally utilized for a combined EVT technique characterized by using a stent retriever with simultaneous aspiration [ 14]. In other patients, contact aspiration using a direct aspiration first-pass technique was adopted as the initial treatment method for EVT [ 32].
Recanalization and clinical outcome assessment
After each pass of the device, on angiography, recanalization status was assessed based on the mTICI score. The FPE was defined as achieving a complete recanalization (mTICI score 3) of the ELVO after a single pass of the thrombectomy device without the use of rescue therapies such as balloon angioplasty, implantation of a stent, or intra-arterial drug administration [ 23]. Post-procedural hemorrhagic complication revealed on brain CT scans performed within 24 hours was regarded as symptomatic intracranial hemorrhage if the NIHSS score increased by 4 points or greater [ 21]. Outcome measures included the NIHSS scores at discharge, 90-day modified Rankin Scale (mRS) scores, and mortality at 90 days. We defined the mRS scores of 0-2 at 90 days as functional independence.
Statistical analysis
All data are expressed as mean┬▒standard deviation or median with interquartile range for continuous variables and the number of patients with percentage for categorical variables, where appropriate. Comparative analyses between patients who achieved FPE and all others regarding baseline characteristics, clinical variables, and clinical outcomes were performed using the StudentŌĆÖs t-test, Mann-Whitney U test, chi-squared test, and FisherŌĆÖs exact test, as appropriate. To identify predictors of FPE, potential predictive factors with p<0.10 in the univariate analysis were entered into the multivariate logistic regression analysis, for which odds ratios (ORs) and 95% confidential intervals (CIs) were provided. All statistical analyses were conducted using SPSS ver. 23.0 software (IBM Corp., Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.
RESULTS
A consecutive series of 110 patients (mean age, 69.5┬▒12.1 years; M : F ratio, 54 : 56) were finally included in the current study. FPE was achieved in 31 patients (28.2%), and the nonFPE group (n=79, 71.8%) comprised mTICI 2b (n=42, 38.2%) and mTICI 3 (n=37, 33.6%) ( Fig. 1).
Differences in characteristics between the FPE group and the non-FPE group
Table 1 summarizes the differences in baseline characteristics and clinical variables between the two groups. No significant differences in demographics, stroke severity, stroke etiology, or use of antithrombotic medications were found in both groups. More patients in the FPE group revealed MCA-M1 occlusion on cerebral angiography than in the non-FPE group (74.2% vs. 60.8%), but there was no statistical significance ( p=0.172).
Pretreatment IVT (p=0.010), DTP interval (p=0.001), use of BGC (p=0.006), and choice of combined technique as a firstline strategy (p=0.099) were found as potential predictive factors of FPE (p<0.10).
Predictive factors of FPE
The results of the multivariate analysis for the predictive factors of FPE are demonstrated in Table 2. Pretreatment IVT (OR, 3.179; 95% CI, 1.025-9.861; p=0.045), DTP interval (OR, 0.959; 95% CI, 0.932-0.987; p=0.004), and the use of BGC (OR, 3.591; 95% CI, 1.231-10.469; p=0.019) were found to be significantly associated with FPE. The adoption of the combined technique as a first-line strategy was not reported as an independent predictor of FPE, while it trended toward significance ( p=0.068).
Outcomes in the FPE versus non-FPE groups
The median procedure time in the FPE group was significantly shorter compared with the non-FPE group (35 vs. 50 minutes, p<0.001). Patients who achieved the FPE had lower median NIHSS scores at discharge (4 vs. 8 points, p<0.001). Furthermore, we noted that significantly higher proportions of patients with FPE had functional independence than those in the non-FPE group (80.6% vs. 50.6%, p=0.002) ( Table 3).
DISCUSSION
The current study revealed independent predictive factors that could have an impact on the occurrence of FPE in patients with ELVO of the anterior circulation who achieved successful reperfusion after EVT. The significant findings of this study were that pretreatment IVT, use of BGC, and shorter DTP intervals were positively associated with FPE. Notably, patients in the FPE group showed significantly shorter procedure times, lower NIHSS scores at discharge, and higher rates of 90-day functional independence. The present study was performed after limiting the patient cohort to those who achieved successful recanalization after EVT. This may show the effects of FPE on clinical outcomes more directly and increase the validity of the predictive factors that we identified.
Since the new concept referred to as the FPE has been proposed as a novel metric of successful EVT [ 35], subsequent studies have highlighted the association between first-pass reperfusion and a higher rate of favorable outcomes [ 8, 16, 23]. According to a previous matched-cohort analysis conducted on 164 patients with anterior circulation ELVO, the favorable outcome was almost doubled in patients who achieved complete recanalization after the first device pass [ 23]. More recently, a retrospective study was conducted to identify the predictors of FPE in a limited cohort after excluding patients who failed thrombectomy (mTICI <2b) using the contact aspiration technique. The authors described the benefits of achieving recanalization on the first pass, demonstrating that good functional outcomes at 90 days were found in 80.3% of patients (53/66) [ 16]. Similarly, in our results, we demonstrated more than 1.5-fold of functional independence in patients who achieved FPE (80.6% vs. 50.6%). According to a recent meta-analysis, patients who underwent EVT after IVT showed a higher probability of obtaining successful recanalization only through a lower number of device passes [ 22]. Presumably, this might be because IVT may dissolve or soften the thrombus, thus making it easier to perform contact aspiration and stent retrieval, thereby facilitating EVT with fewer passes of the thrombectomy device [ 10, 22]. These results support the current national guidelines for administering IV tPA to eligible patients [ 24]. It cannot be overlooked, however, that the incidence of distal embolization may increase because of clot fragmentation induced by IVT [ 11]. But there is also a contradictory study showing that distal embolization had no significant correlation with pretreatment IVT [ 13]. Further studies are therefore warranted to assess the net clinical benefit of pretreatment IVT, therefore being mandatory to examine whether the effect of pretreatment IVT in softening the thrombus could offset the risk of distal embolization. To date, the efficacy of BGC in patients with ELVO undergoing EVT has been well described in the literature [ 6, 16, 20, 35]. In our results, patients who achieved FPE had a higher proportion of BGC use (80.6% vs. 54.4% in non-FPE patients). In addition, we found that BGC use had a significant positive correlation with a higher rate of FPE in multivariate analysis. This concurs with a recent meta-analysis demonstrating that the utilization of BGC during EVT had a significant correlation with excellent angiographic outcomes, including a higher proportion of successful reperfusion and FPE [ 6]. In detail, patients in whom BGC was used versus those in whom it was not used have disparities in mTICI 2b/3 and mTICI 3 rates of 12% and 20%, respectively. This indicates a potential advantage of BGC : the use of BGC was effective in inhibiting clot fragmentation and distal embolization through antegrade flow arrest during the clot retrieval procedure [ 20]. In the aspect of required force to clot retrieval, the inflation of the BGC might markedly decrease the impaction force arising from the pressure gradient across the thrombus and proximal systemic blood pressure bearing on the clot. This can boost the effectiveness of thrombectomy devices [ 16, 34]. In support of the aforementioned theoretical basis, several recent studies show that BGC utilization was an independent predictor of FPE, which is similar to our results [ 16, 35]. Recently, several studies have been conducted on the interaction between successful reperfusion and time-related variables [ 5, 8, 9, 15, 27]. According to a recent meta-analysis, the chance of successful recanalization decreases significantly with a longer hospital arrival-to-groin puncture time corresponding to the DTP interval defined in the current study [ 5]. Moreover, a recent retrospective study on 257 patients showed that a decreased DTP interval improved the odds of first-pass reperfusion (mTICI 2b/3) [ 27]. Presumably, this might be due to time-dependent changes in the thrombus : its components become abundant in fibrin and transform into a denser and more compact structure. Therefore, a greater number of passes of the EVT device is required to achieve successful recanalization [ 9, 15]. Interestingly, the other time-related variables, OTD and OTP intervals, had no significant correlation with FPE in our results, which is in line with previous studies [ 5, 8, 27]. This might be attributed to the variable reliability of the exact time point of stroke onset compared with the accurately recorded hospital arrival time. In this context, we highlight the importance of rapid patient triage and initiation of EVT without delay to shorten the DTP interval as a modifiable factor. This is essential for optimizing recanalization, although there was only approximately 15-minute difference in the absolute value of the DTP interval between the two groups in our series. Although EVT with a stent retriever is the standard treatment for AIS due to ELVO [ 24], the potential synergistic effect of the combined technique (stent retriever plus simultaneous aspiration) in association with FPE has been recently described [ 8, 19, 27]. The combined technique allows continuous negative-pressure aspiration at a location proximal to the thrombus during stent retrieval, thus making it possible to achieve a higher proportion of successful reperfusion. Contrary to previous studies, we failed to present a statistically significant correlation between the combined technique and FPE, whereas a trend for FPE was observed in the univariate analysis. This result might have originated from the small series of enrolled patients in the present study. There was also no balanced distribution of adoption of the first-line thrombectomy strategy because it is usual for a single institution to prefer one technique over another. Further large-scale multi-center studies comparing various thrombectomy techniques will help to define the ideal technical strategy to increase the rate of first-pass recanalization. Our results cannot be generalized. The limitations of the current study are as follows : first, we included a relatively small series of patients in the current single-center retrospective study. Further investigations designed with multi-center larger cohorts are warranted to generalize our results. Second, we failed to assess potentially relevant factors contributing to the achievement of FPE, such as clot histology (thrombus composition) [ 17], size of stent retriever [ 29], and carotid tortuosity [ 33]. Nonetheless, our results deserve special attention; the homogeneity of our cohort (mTICI Ōēź2b) in contrast to the heterogeneous cohort (including mTICI less than 2b) included in previous studies may increase the validity of predictive factors that we identified.
CONCLUSION
We identified predictive factors of FPE in patients with ELVO of the anterior circulation who achieved successful reperfusion following EVT. Pretreatment IVT, use of BGC, and a shorter DTP interval were positively associated with FPE, increasing the chance of acquiring better clinical outcomes.
Fig.┬Ā1.
Flowchart of patient enrollment. ELVO : emergent large vessel occlusion, EVT : endovascular thrombectomy, MCA : middle cerebral artery, ACA : anterior cerebral artery, FPE : first-pass effect, mTICI : modified Thrombolysis in Cerebral Infarction. 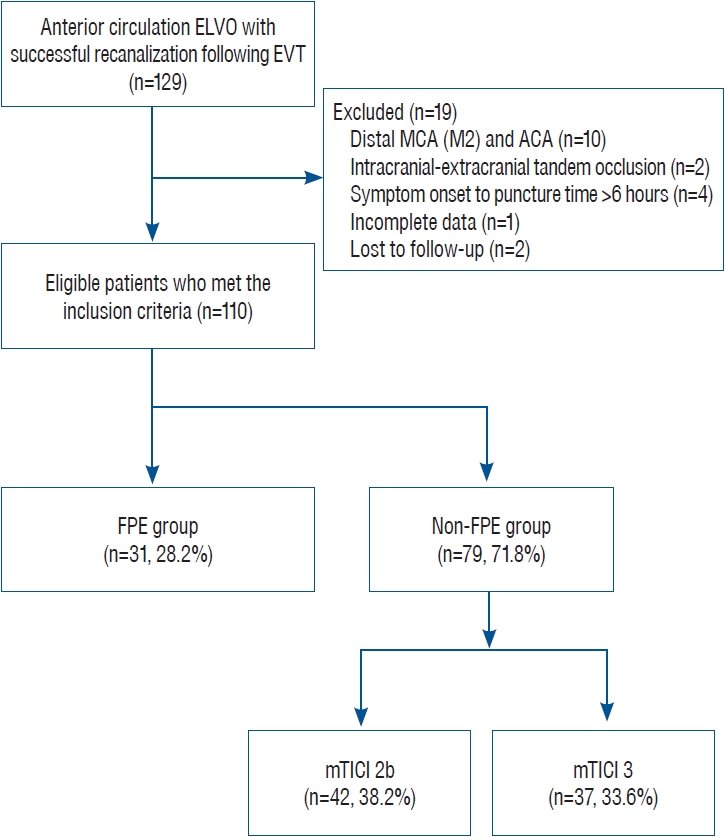
Table┬Ā1.
Comparison of baseline characteristics and clinical variables between the FPE and non-FPE groups
|
Variable |
FPE (n=31) |
Non-FPE (n=79) |
p-value |
|
Male |
15 (48.4) |
39 (49.4) |
0.928 |
|
Age (years) |
67.7┬▒10.8 |
70.2┬▒12.4 |
0.300 |
|
Medical history |
|
|
|
|
Hypertension |
16 (51.6) |
40 (50.6) |
0.928 |
|
Diabetes mellitus |
6 (19.4) |
23 (29.1) |
0.275 |
|
Atrial fibrillation |
9 (29.0) |
23 (29.1) |
0.993 |
|
Dyslipidemia |
9 (29.0) |
27 (34.2) |
0.609 |
|
Coronary heart disease |
8 (25.8) |
19 (24.1) |
0.849 |
|
Previous ischemic stroke |
3 (9.7) |
14 (17.7) |
0.249 |
|
Antiplatelet medication |
12 (38.7) |
25 (31.6) |
0.485 |
|
Anticoagulant medication |
5 (16.1) |
16 (20.3) |
0.624 |
|
NIHSS score on admission |
15 (10-19) |
17 (11-20) |
0.200 |
|
Stroke etiology |
|
|
|
|
Large-artery atherosclerosis |
6 (19.4) |
21 (26.6) |
0.433 |
|
Cardioembolic |
23 (74.2) |
54 (68.4) |
0.552 |
|
Other or unknown |
2 (6.5) |
4 (5.1) |
0.775 |
|
ASPECTS |
9 (8-9) |
8 (8-9) |
0.231 |
|
Site of occlusion |
|
|
0.172 |
|
MCA-M1 |
23 (74.2) |
48 (60.8) |
|
|
Intracranial ICA |
8 (25.8) |
31 (39.2) |
|
|
Clot burden score |
7.1┬▒0.9 |
6.9┬▒1.1 |
0.214 |
|
Pretreatment IVT |
26 (83.9) |
48 (60.8) |
0.010*
|
|
Time metrics |
|
|
|
|
Onset-to-door (minutes) |
193.7┬▒58.5 |
195.1┬▒56.7 |
0.907 |
|
|
Median (IQR) |
215 (152.5-237.5) |
200 (157.5-240) |
|
|
Door-to-puncture (minutes) |
58.9┬▒17.1 |
73.2┬▒21.2 |
0.001*
|
|
|
Median (IQR) |
55 (45 -67.5) |
70 (60-85) |
|
|
Onset-to-puncture (minutes) |
252.6┬▒56.1 |
268.4┬▒52.6 |
0.166 |
|
|
Median (IQR) |
265 (210-295) |
280 (242.5-302.5) |
|
|
Use of BGC |
25 (80.6) |
43 (54.4) |
0.006*
|
|
First-line strategy |
|
|
|
|
Stent retriever |
14 (45.2) |
49 (62.0) |
0.110 |
|
Contact aspiration |
6 (19.4) |
15 (19.0) |
0.965 |
|
Combined technique |
11 (35.5) |
15 (19.0) |
0.099*
|
Table┬Ā2.
Multivariate analysis for independent predictive factors of FPE
|
Variable |
Odds ratio (95% CI) |
p-value |
|
FPE vs. non-FPE |
|
|
|
ŌĆāPretreatment IVT |
3.179 (1.025-9.861) |
0.045*
|
|
ŌĆāDoor-to-puncture, per 1-minute increase |
0.959 (0.932-0.987) |
0.004*
|
|
ŌĆāUse of BGC |
3.591 (1.231-10.469) |
0.019*
|
|
ŌĆāCombined technique |
2.723 (0.928-7.985) |
0.068 |
Table┬Ā3.
Comparison of procedure time and clinical outcomes in the FPE versus non-FPE groups
|
Variable |
FPE (n=31) |
Non-FPE (n=79) |
p-value |
|
Procedure time (minutes) |
35 (27.5-42.5) |
50 (40-57.5) |
<0.001*
|
|
NIHSS score at discharge time (minutes) |
4 (1.5-6.5) |
8 (6-10) |
<0.001*
|
|
Functional independence, mRS 0-2 |
25 (80.6) |
40 (50.6) |
0.002*
|
|
Mortality at 90 days |
2 (6.5) |
8 (10.1) |
0.551 |
|
Symptomatic ICH |
2 (6.5) |
10 (12.7) |
0.352 |
References
1. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al : Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 24 : 35-41, 1993   2. Adcock AK, Schwamm LH, Smith EE, Fonarow GC, Reeves MJ, Xu H, et al : Trends in use, outcomes, and disparities in endovascular thrombectomy in US patients with stroke aged 80 years and older compared with younger patients. JAMA Netw Open 5 : e2215869, 2022    3. Baek JH, Kim BM, Heo JH, Nam HS, Kim YD, Park H, et al : Number of stent retriever passes associated with futile recanalization in acute stroke. Stroke 49 : 2088-2095, 2018   4. Berkhemer OA, Fransen PS, Beumer D, Van Den Berg LA, Lingsma HF, Yoo AJ, et al : A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372 : 11-20, 2015  5. Bourcier R, Goyal M, Liebeskind DS, Muir KW, Desal H, Siddiqui AH, et al : Association of time from stroke onset to groin puncture with quality of reperfusion after mechanical thrombectomy: a meta-analysis of individual patient data from 7 randomized clinical trials. JAMA Neurol 76 : 405-411, 2019    6. Brinjikji W, Starke RM, Murad MH, Fiorella D, Pereira VM, Goyal M, et al : Impact of balloon guide catheter on technical and clinical outcomes: a systematic review and meta-analysis. J Neurointerv Surg 10 : 335-339, 2018   7. Dargazanli C, Consoli A, Barral M, Labreuche J, Redjem H, Ciccio G, et al : Impact of modified TICI 3 versus modified TICI 2b reperfusion score to predict good outcome following endovascular therapy. AJNR Am J Neuroradiol 38 : 90-96, 2017    8. Di Maria F, Kyheng M, Consoli A, Desilles JP, Gory B, Richard S, et al : Identifying the predictors of first-pass effect and its influence on clinical outcome in the setting of endovascular thrombectomy for acute ischemic stroke: results from a multicentric prospective registry. Int J Stroke 16 : 20-28, 2021    9. Duffy S, McCarthy R, Farrell M, Thomas S, Brennan P, Power S, et al : Per-pass analysis of thrombus composition in patients with acute ischemic stroke undergoing mechanical thrombectomy. Stroke 50 : 1156-1163, 2019   10. Fischer U, Kaesmacher J, Mendes Pereira V, Chapot R, Siddiqui AH, Froehler MT, et al : Direct mechanical thrombectomy versus combined intravenous and mechanical thrombectomy in large-artery anterior circulation stroke: a topical review. Stroke 48 : 2912-2918, 2017   11. Flint AC, Avins AL, Eaton A, Uong S, Cullen SP, Hsu DP, et al : Risk of distal embolization from tPA (tissue-type plasminogen activator) administration prior to endovascular stroke treatment. Stroke 51 : 2697-2704, 2020  12. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al : Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387 : 1723-1731, 2016   13. Guedin P, Larcher A, Decroix JP, Labreuche J, Dreyfus JF, Evrard S, et al : Prior IV thrombolysis facilitates mechanical thrombectomy in acute ischemic stroke. J Stroke Cerebrovasc Dis 24 : 952-957, 2015   14. Humphries W, Hoit D, Doss VT, Elijovich L, Frei D, Loy D, et al : Distal aspiration with retrievable stent assisted thrombectomy for the treatment of acute ischemic stroke. J Neurointerv Surg 7 : 90-94, 2015   15. Jolugbo P, Ari├½ns RAS : Thrombus composition and efficacy of thrombolysis and thrombectomy in acute ischemic stroke. Stroke 52 : 1131-1142, 2021    16. Kang DH, Kim BM, Heo JH, Nam HS, Kim YD, Hwang YH, et al : Effects of first pass recanalization on outcomes of contact aspiration thrombectomy. J Neurointerv Surg 12 : 466-470, 2020   18. Kleine JF, Wunderlich S, Zimmer C, Kaesmacher J : Time to redefine success? TICI 3 versus TICI 2b recanalization in middle cerebral artery occlusion treated with thrombectomy. J Neurointerv Surg 9 : 117-121, 2017   19. Lapergue B, Labreuche J, Blanc R, Marnat G, Consoli A, Rodesch G, et al : Combined use of contact aspiration and the stent retriever technique versus stent retriever alone for recanalization in acute cerebral infarction: the randomized ASTER 2 study protocol. J Neurointerv Surg 12 : 471-476, 2020   20. Lee DH, Sung JH, Kim SU, Yi HJ, Hong JT, Lee SW : Effective use of balloon guide catheters in reducing incidence of mechanical thrombectomy related distal embolization. Acta Neurochir (Wien) 159 : 1671-1677, 2017    21. Lee YB, Yoon W, Lee YY, Kim SK, Baek BH, Kim JT, et al : Predictors and impact of hemorrhagic transformations after endovascular thrombectomy in patients with acute large vessel occlusions. J Neurointerv Surg 11 : 469-473, 2019   22. Mistry EA, Mistry AM, Nakawah MO, Chitale RV, James RF, Volpi JJ, et al : Mechanical thrombectomy outcomes with and without intravenous thrombolysis in stroke patients: a meta-analysis. Stroke 48 : 2450-2456, 2017   23. Nikoubashman O, Dekeyzer S, Riabikin A, Keulers A, Reich A, Mpotsaris A, et al : True first-pass effect: first-pass complete reperfusion improves clinical outcome in thrombectomy stroke patients. Stroke 50 : 2140-2146, 2019   24. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al : 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. stroke 49 : e46-e110, 2018  25. Puetz V, Dzialowski I, Hill MD, Subramaniam S, Sylaja PN, Krol A, et al : Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: the clot burden score. Int J Stroke 3 : 230-236, 2008    26. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al : Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 372 : 2285-2295, 2015   27. Schmidt RF, Sweid A, Mouchtouris N, Velagapudi L, Chalouhi N, Gooch MR, et al : Predictors of first-pass reperfusion for mechanical thrombectomy in acute ischemic stroke. Clin Neurol Neurosurg 219 : 107314, 2022   28. Seker F, Pfaff J, Wolf M, Ringleb PA, Nagel S, Sch├Čnenberger S, et al : Correlation of thrombectomy maneuver count with recanalization success and clinical outcome in patients with ischemic stroke. AJNR Am J Neuroradiol 38 : 1368-1371, 2017    29. Serna Candel C, Aguilar P├®rez M, B├żzner H, Henkes H, Hellstern V : First-pass reperfusion by mechanical thrombectomy in acute M1 occlusion: the size of retriever matters. Front Neurol 12 : 679402, 2021   30. Shigeta K, Suzuki K, Matsumaru Y, Takeuchi M, Morimoto M, Kanazawa R, et al : Intravenous alteplase is associated with first pass effect in stent-retriever but not ADAPT thrombectomy : post hoc analysis of the SKIP study. Clin Neuroradiol 32 : 153-162, 2022    31. Tsang COA, Cheung IHW, Lau KK, Brinjikji W, Kallmes DF, Krings T : Outcomes of stent retriever versus aspiration-first thrombectomy in ischemic stroke: a systematic review and meta-analysis. AJNR Am J Neuroradiol 39 : 2070-2076, 2018    32. Turk AS, Frei D, Fiorella D, Mocco J, Baxter B, Siddiqui A, et al : ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg 10( Suppl 1):i4-i7, 2018   35. Zaidat OO, Castonguay AC, Linfante I, Gupta R, Martin CO, Holloway WE, et al : First pass effect: a new measure for stroke thrombectomy devices. Stroke 49 : 660-666, 2018  
|
|

















