Kim, Byun, Lee, and Hong: A Potential Risk of Radiation-Induced Cavernous Malformations Following Adjuvant Gamma Knife Radiosurgery for Mesial Temporal Lobe Epilepsy
Abstract
Objective
Several clinical studies have explored the feasibility and efficacy of radiosurgical treatment for mesial temporal lobe epilepsy, but the long-term safety of this treatment has not been fully characterized. This study aims to report and describe radiation-induced cavernous malformation as a delayed complication of radiosurgery in epilepsy patients.
Methods
The series includes 20 patients with mesial temporal lobe epilepsy who underwent Gamma Knife radiosurgery (GKRS). The majority received a prescribed isodose of 24 Gy as an adjuvant treatment after anterior temporal lobectomy.
Results
In this series, we identified radiation-induced cavernous malformation in three patients, resulting in a cumulative incidence of 18.4% (95% confidence interval, 6.3% to 47.0%) at an 8-year follow-up. These late sequelae of vascular malformation occurred between 6.9 and 7.6 years after GKRS, manifesting later than other delayed radiation-induced changes, such as radiation necrosis. Neurological symptoms attributed to intracranial hypertension were present in those three cases involving cavernous malformation. Of these, two cases, which initially exhibited an insufficient response to radiosurgery, ultimately demonstrated seizure remission following the successful microsurgical resection of the cavernous malformation.
Conclusion
All things considered, the development of radiation-induced cavernous malformation is not uncommon in this population and should be acknowledged as a potential long-term complication. Microsurgical resection of cavernous malformation can be preferentially considered in cases where the initial seizure outcome after GKRS is unsatisfactory.
Key Words: Cerebral cavernous malformation · Gamma Knife radiosurgery · Mesial temporal lobe epilepsy · Hippocampal sclerosis · Anterior temporal lobectomy.
INTRODUCTION
Stereotactic radiosurgery is currently recognized as a viable treatment option in patients with intractable epilepsy as an alternative to conventional epilepsy surgery. For mesial temporal lobe epilepsy (MTLE) with hippocampal sclerosis, radiosurgery has demonstrated effectiveness comparable to anterior temporal lobectomy (ATL) [ 2, 4, 19], although its non-inferiority was not established by the outcomes within 3 years from a randomized controlled study [ 3]. The lower rate of neuropsychological consequences after radiosurgery is also considered a major advantage over standard ATL [ 16]. Previous research has also explored the application of radiosurgery as an adjuvant treatment for refractory MTLE after unsuccessful ATL [ 14]. This minimally invasive, incisionless radiosurgical procedure could help reduce operative burdens for epilepsy patients requiring open surgery. However, the long-term safety of this treatment modality for epilepsy has not been exhaustively defined. Unlike other treatments, radiation exposure can result in unanticipated complications due to various radiobiological processes over extended periods [ 5, 11]. Thus, continual re-evaluation of longterm safety is imperative. In this context, we emphasize the potential risk of radiation-induced cavernous malformations (RICM)—a recognized but infrequent late complication following radiosurgery—which appear to occur more incidentally within a certain population of epilepsy patients who have received radiosurgery.
MATERIALS AND METHODS
Study approval
This research was approved by the Institutional Review Board of Asan Medical Center (project number 2023-1154). All methods were conducted in accordance with relevant guidelines and regulations.
Study population
This retrospective study included patients with MTLE who underwent Gamma Knife radiosurgery (GKRS) at a single tertiary-level referral epilepsy center between 2001 and 2021. We excluded patients who underwent radiosurgery for the treatment of brain tumors or arteriovenous malformations, those who underwent radiosurgery for therapeutic targets other than mesial temporal structures, such as hypothalamic hamartoma, periventricular focal nodular heterotopia, or corpus callosum, and those who lost to follow-up before 2 years from radiosurgery.
Radiosurgical treatment
All treatment strategies were determined by a multidisciplinary epilepsy team comprised of adult and pediatric epileptology specialists, neurosurgeons, and neuroradiologists. Patients received either primary or adjuvant radiosurgery, depending on their clinical conditions. Adjuvant GKRS was indicated for patients with Engel class III or IV seizure outcomes after ATL.
For radiosurgery, the Leksell Gamma-Knife Models B, C, and Perfexion (Elekta AB, Stockholm, Sweden) were utilized. In each case, 1.0 mm-sectioned post-contrast three dimensional T1-weighted magnetic resonance imagings (MRIs) and 2.0 mm-sectioned coronal T2-weighted MRIs were acquired for treatment planning. The therapeutic target was planned by experienced epilepsy neurosurgeons according to the hypothesis of epileptogenic regions of each patient. In patients who underwent GKRS as a primary treatment without surgery, the main targets were the hippocampus and the amygdaloid complex. In cases where radiosurgery was used as a secondary adjuvant treatment following a failed first resective surgery, the target volume included any remnant mesial temporal structures and adjacent regions, including the posterior parts of the parahippocampal gyrus and the fusiform gyrus. The radiation doses were typically prescribed at 24 Gy on a 50% isodose line. Organ-at-risk (OAR) constraints were adhered to limit the maximum exposure to 12 Gy for the brainstem and 8 Gy for the optic apparatus.
Outcome assessment
Patients underwent follow-up structural MRIs within 1 to 2 years post-GKRS, with additional scans performed thereafter as clinically indicated. Post-treatment changes observed in the follow-up MRIs after GKRS were assessed and described by both neurosurgeons and neuroradiologists. Delayed radiationinduced changes, including early T2 hyperintense signal changes and other late structural changes, were identified [ 23]. The late delayed changes encompassed radiation necrosis, tumefactive cyst formation, and vascular malformations. Cavernous malformations were diagnosed in cases presenting distinct imaging findings, such as popcorn appearance and hemosiderin deposition, which are not typical in radiation necrosis. For cases that developed symptomatic complications, the associated clinical details were meticulously reviewed. Patients were maintained on antiseizure medications (ASMs) and regularly followed up by epileptology specialists. The efficacy of seizure control after the treatment was assessed using the Engel classification, categorizing outcomes as either favorable (Engel I/II) or unfavorable (Engel III/IV). Those classified as Engel Ia or Ib were deemed to have achieved seizure remission [ 3]. For cases with refractory seizures, referral to the multidisciplinary epilepsy team was considered.
Statistical consideration
Descriptive statistics were reported as median values with interquartile ranges (IQR) unless otherwise specified. The groupwise comparison between subgroups was conducted using the Mann-Whitney test, with a p-value less than 0.05 considered statistically significant. The cumulative incidence of time-to-event outcome variables was estimated using the Kaplan-Meier method and presented with 95% confidence intervals (CIs).
RESULTS
Baseline characteristics
In this series, a total of 20 cases were reviewed ( Table 1). The median age was 25.5 years (IQR, 31.0-42.8), with males constituting 70.0% (n=14). The age at the onset of seizures and the disease duration were 11.5 years (IQR, 9.8-23.3) and 12.0 years (IQR, 8.8-17.3), respectively. Most patients (90.0%) underwent adjuvant radiosurgery after ATL for MTLE, except for two patients who received GKRS as the primary treatment for MTLE. The median duration of the disease, prior to the initial surgical intervention, was 12.0 years (IQR, 8.8-17.3). For those who underwent adjuvant GKRS following ATL, the median time interval between ATL and GKRS was 5.5 years (IQR, 2.4-12.4). The seizures originated from the left hemisphere in half of the patients. Regarding the underlying structural etiology, 13 cases had hippocampal sclerosis, three were diagnosed with a combined pathology of hippocampal sclerosis and cortical dysplasia, and one exhibited cortical dysplasia only. The remaining three cases that underwent primary GKRS without open surgery were not histopathologically diagnosed but exhibited typical radiological findings suggestive of hippocampal sclerosis. The most common seizure types were focal seizures in a half, while the other half presented focal to bilateral seizures.
Seizure control
GKRS was the primary intervention for two patients, while the others received adjuvant GKRS post-ATL. In the latter group, 15 patients were categorized under Engel Ⅲ, and the remaining three under Engel Ⅳ after ATL. In terms of the radiosurgical treatment parameters, the target region predominantly included the hippocampus and amygdala in seven cases, the fusiform gyrus in 10 cases, and the parahippocampal gyrus in three cases, respectively. The treatment employed a median of 7 isocenters (IQR, 4-11), targeting a median volume of 4.6 cm3 (IQR, 3.4-6.3) at the prescribed 50% isodose of 24 Gy.
Before undergoing GKRS treatment, six patients experienced at least one seizure day monthly, five had 6-12 seizure days annually, another five had 3-6 seizure days annually, and the remaining four had fewer than three seizure days a year. After the GKRS procedure, 70.0% (n=14) of the patients achieved favorable seizure control. Half of these respondents showed an early response within the first 4 years, while the remainder presented a late response after 4 years. Conversely, the remaining cases (n=6) did not demonstrate favorable outcomes. After GKRS, 40.0% (n=8) of the patients had their ASMs reduced with successful seizure control, whereas the others (n=12) did not.
Incidence of radiation-induced cavernous malformation
During a median clinical follow-up of 14.0 years (IQR, 9.2-16.1), delayed radiation-induced changes were identified in eight patients through post-GKRS MRIs. These changes, which manifested at a median of 5.3 years (IQR, 4.5-6.7), included radiation necrosis in six patients and tumefactive cyst formation in two patients. Of note, three cases were diagnosed with RICM, all of which occurred as a result of adjuvant GKRS after ATL. The development of RICMs was observed later than other radiation-induced changes, with the time to event ranging from 6.9 to 7.6 years post-GKRS ( Fig. 1). By the 8-year point, the cumulative incidence of RICM had surpassed 18.4% (95% CI, 6.3% to 47.0%). The three cases that developed RICM had undergone standard GKRS procedures similar to the others. Their radiosurgical treatment parameters, as well as the total volumes receiving each dose of radiation, did not show notable differences from the remaining cases. Specifically, the gradient index was 2.73 (IQR, 2.72-2.85) vs. 2.88 (IQR, 2.73-2.97) (p=0.689), the maximum dose was 48.2 Gy (IQR, 48.1-48.7) vs. 48.9 Gy (IQR, 48.4-49.0) (p=0.790), the OAR constraint was 9.8 Gy (IQR, 8.5-10.6) vs. 10.6 Gy (IQR, 8.1-12.2) (p=0.491), the dose coverage was 3.6 cm3 (IQR, 3.0-3.7) vs. 5.3 cm3 (IQR, 4.2-6.3) (p=0.118) at 24 Gy, 2.0 cm3 (IQR, 1.7-2.2) vs. 2.8 cm3 (IQR, 2.3-3.3) (p=0.258) at 30 Gy, and 0.77 cm3 (IQR, 0.73-0.82) vs. 1.18 cm3 (IQR, 0.62-1.32) (p=0.358) at 36 Gy. Additionally, baseline variables such as age, sex, onset and duration of seizures, and seizure type did not exhibit associations with the risk of RICM occurrence.
Clinical course and treatment of radiation-induced cavernous malformation
Among the three patients diagnosed with RICM, one patient had an initial unfavorable seizure outcome (Engel III), while the other two patients had equivocal seizure outcomes (Engel Ic). Their seizures had gradually improved, with occasional increases noted during periods of delayed radiation-induced changes, similar to observations in other typical cases. At the time RICM manifested, these patients exhibited neurological symptoms, such as progressive headache, dizziness, or blurred vision, indicating increased intracranial pressure. Radiological findings revealed the presence of a heterogeneously enhancing mass along with large cyst formation or enlargement of the adjacent temporal horn, which were consistent with cavernous malformation ( Fig. 2). Regarding the treatment, two cases underwent microsurgical resection of RICM to resolve neurological deterioration. These surgical interventions resulted in seizure remission, which showed more favorable when compared to the initial post-GKRS results. Conversely, one patient who initially exhibited a favorable response to radiosurgery chose a conservative approach without opting for surgery. During the follow-up period, the cyst associated with the RICM lesion spontaneously decreased in size, leading to an improvement in headaches and other symptoms.
Case 1
A 29-year-old male underwent a left ATL 7 years ago for medically refractory MTLE with hippocampal sclerosis and focal cortical dysplasia. Initially, he experienced a significant reduction in seizures and successfully reduced his ASMs. However, 2 years after the ATL, his seizure recurred, with a frequency exceeding three times a year, despite an escalation in ASMs. The patient subsequently underwent GKRS targeting on a target volume of 2.4 cm 3 with 24 Gy ( Fig. 2A). However, the patient’s seizures did not significantly reduce even after GKRS (Engel III). Seven years post-GKRS, the patient developed new severe headaches and dizziness. An MRI scan revealed a 4 cm-sized hemorrhagic cystic mass within the previously irradiated field ( Fig. 2B). A revisional craniotomy was performed, xanthochromic fluid was drained from the cyst, and the hemorrhagic mass was completely removed. The mass was fed by the inferior temporal artery and was connected to the tail of the hippocampus and the posterior part of the parahippocampal gyrus ( Fig. 2C). After microsurgical resection, the patient remained free from convulsive seizures, only experiencing occasional aura once a month (Engel Ib). Histopathological examination revealed cavernous malformation with sclerosing hematoma, demonstrating dilated and thin-walled vascular spaces ( Fig. 2D).
Case 2
A 16-year-old male underwent a right ATL 9 years ago for medically refractory MTLE with hippocampal sclerosis. Initially, he achieved seizure remission and was able to discontinue ASMs 2 years post-ATL. However, he experienced a recurrence of intractable seizures despite the reintroduction of four ASMs. He received GKRS on a target volume of 3.6 cm 3 with 24 Gy ( Fig. 2E), and his disabling seizures disappeared 5 years post-GKRS (Engel Ic). However, 7 years after GKRS, the patient presented with progressive visual disturbance. An MRI revealed a 3 cm-sized enhancing mass within the previously irradiated field, accompanied by the interval development of hydrocephalus ( Fig. 2F). A revisional craniotomy for tumor resection was performed for both decompression and histopathological confirmation. The mass, located inferior to the choroid plexus of the right temporal horn, was totally removed based on gross inspection ( Fig. 2G). The patient did not experience further neurological deficits and became entirely seizure-free following ASM reduction (Engel Ia). Histopathological examination of the surgical specimen confirmed the organizing hematoma to be consistent with cavernous malformation ( Fig. 2H).
Case 3
A 32-year-old male underwent a left ATL 14 years ago for medically refractory MTLE with a combined pathology of hippocampal sclerosis and focal cortical dysplasia. He received GKRS for a target volume of 3.8 cm 3 with 24 Gy ( Fig. 2I). The patient’s seizures had been well-controlled with ASMs (Engel Ic). However, 7 years post-GKRS, he presented with headache. An MRI exhibited an organizing hematoma displaying mixed signal intensities due to various stages of blood products, along with large cyst formation ( Fig. 2J). While these radiological features were consistent with features commonly observed in cavernous malformation ( Fig. 2L), the patient opted for a conservative approach observation without surgery, as his symptoms were tolerable. Over the subsequent 3 years of follow-up, the cystic portion gradually decreased in size without additional intervention ( Fig. 2K).
DISCUSSION
In this report, we highlight a substantial risk of RICM as a delayed complication of GKRS, which was observed in at least 6.3% of our MTLE case series. Despite the emergence of radiosurgery as an alternative treatment for epilepsy over the past decades, RICM has been scarcely reported in this population [ 24]. The incidence of RICM has been reported with increased rates after cranial irradiation with higher doses. In a long-term follow-up cohort study involving a specific pediatric population indicated for radiation therapy, RICM was found in all patients who received 18 to 36 Gy, in half of those who received 6 to 12 Gy, and in none of those who were not exposed to radiation therapy [ 13]. The latency period for the development of RICM can reach 8 years [ 7, 17, 18]. Such delayed radiation-induced changes have been observed with an elevated risk where brain parenchymal tissue is involved within the targeted radiation delivery field during GKRS [ 12]. In an MTLE series treated with GKRS, the histopathological evaluation revealed microbleeds and neoangiogenesis in over half of the patients [ 22]. These patients proceeded to ATL after primary GKRS proved ineffective. The current radiosurgical treatment for MTLE is characterized by the administration of a marginal isodose of 24 Gy, with a maximal dose up to 48 Gy, targeting a considerable volume of non-neoplastic brain parenchymal tissue [ 16]. Treatments using a lower dose of 20 Gy or below have not yielded sufficient seizure outcome [ 2, 10, 20], and a dose as low as 15 Gy has failed to produce histopathological radiation-induced changes [ 6]. Considering the substantial resemblance in radiation doses between our study and previous investigations [ 3, 4], factors beyond radiation exposure, such as the intricacies of radiosurgical target planning and specific clinical contexts, may also exert an influence on the incidence of RICM. Notably, our study population predominantly consisted of patients who had undergone adjuvant GKRS as part of their treatment for MTLE after ATL. Existing literature indicated a heightened occurrence of RICM in specific patient populations, implying that postoperative alterations compromising cerebral venous flow might render them more susceptible to radiobiological effects and potentially precipitate the development of vascular malformations [ 15]. The radiation effect to the mesial temporal regions can be more pronounced in cases with prior surgical resection that result in subsequent changes in neuroglial tissue and microvasculature. In the context of GKRS for MTLE following initial ATL, we speculate that the complex interactions between this radiation effect on brain parenchymal tissue and surgical changes may contribute to the development of RICM. However, it is important to note that an in-depth exploration into the relationship between the development of RICM and various clinical factors was not feasible in this series due to the limited sample size, necessitating further investigation to draw more conclusive insights. For patients with epilepsy, the development of RICM could be one of the most troublesome late sequelae following radiosurgery due to its propensity for repetitive hemorrhage and high epileptogenic potential [ 8]. Previous literature has demonstrated that RICM carries twice the hemorrhagic risk as nonradiation lesions [ 7] and commonly presents with seizure [ 17]. This may contribute to suboptimal seizure outcomes from radiosurgery in the management of epilepsy. In this context, re-irradiation using radiosurgery or the wait-and-see strategy may not be sufficient as an effective therapeutic approach for symptomatic RICM. We observed improved seizure outcomes in patients with RICM who underwent microsurgical resection, suggesting this may be a preferable treatment option when initial outcomes from radiosurgery are unsatisfactory. Given the high incidence of RICMs and their epileptogenic potential, practitioners should not overlook the significance of these late radiobiological consequences. Recently, other ablative treatment modalities, such as MR-guided stereotactic laser ablation [ 9] and high-intensity focused ultrasound surgery [ 1], have been rigorously explored for their possible applications in epilepsy. These incisionless or minimally invasive novel techniques might become useful for various functional lesioning procedures once substantial evidence for long-term safety and efficacy is established. Until then, functional radiosurgery may continue to serve as an alternative treatment for those who are not suitable candidates for open surgery. The application of functional radiosurgery to other disease entities, such as movement disorders and psychiatric disorders, has also gained growing interest [ 21]. However, the delivery of high radiation doses to non-neoplastic intracranial structures could pose a similar potential risk of delayed vascular complications. From this perspective, it is crucial to recognize that RICM should be considered as a potential late complication of radiosurgery, emphasizing the need for long-term imaging follow-up after the treatment. Future investigations should include a discussion of the optimal procedural factors to minimize the risk of RICM following radiosurgery.
CONCLUSION
The development of RICM is not uncommon among patients who have received GKRS for MTLE. Practitioners should be aware of its potential as a long-term complication. Microsurgical resection of cavernous malformation has shown to be effective in enhancing seizure outcomes, particularly in cases where the initial results after GKRS are unsatisfactory.
Acknowledgements
The authors would like to extend their deep admiration and gratitude to Jung Kyo Lee, a senior functional neurosurgeon, for his substantial contribution to this institution’s epilepsy program. This study was presented at the 63rd Annual Meeting of the Korean Neurosurgical Society in Seoul, Korea.
Fig. 1.
Delayed radiation-induced changes following GKRS for MTLE. Dotted lines indicate the 95% CIs. Early T2 hyperintense changes were evident in the follow-up MRI, conducted 1-year post-GKRS in this series. Late delayed radiation-induced changes, such as radiation necrosis and tumefactive cyst formation, were manifested in eight cases at a median of 5.3 years (IQR, 4.5-6.7). RICMs developed in a subsequent period, resulting in a cumulative incidence of 18.4% (95% CI, 6.3% to 47.0%) at the 8-year time point. GKRS : Gamma Knife radiosurgery, MTLE : mesial temporal lobe epilepsy, CI : confidence interval, MRI : magnetic resonance imaging, RICM : radiation-induced cavernous malformations, IQR : interquartile range. 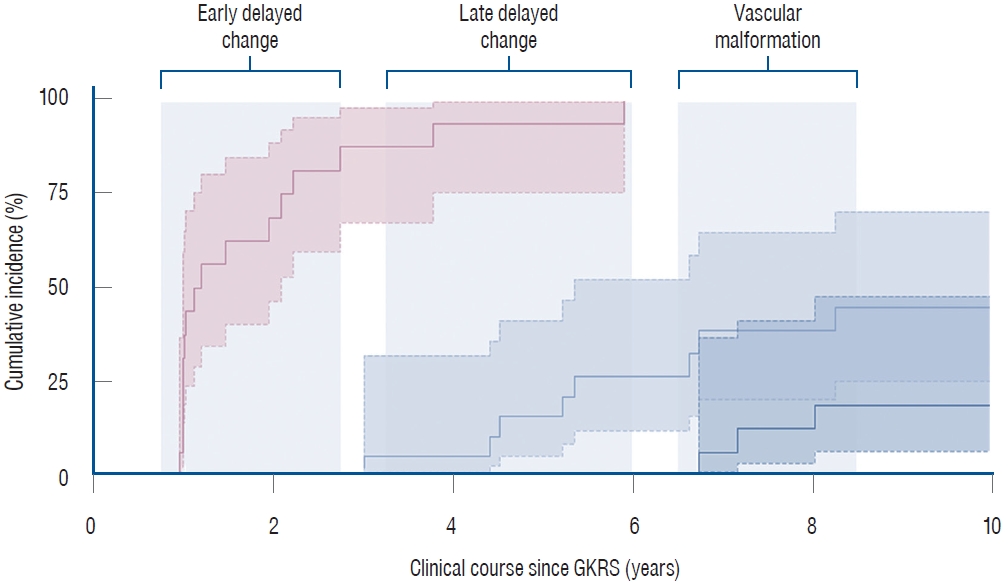
Fig. 2.
Cases presenting RICM after adjuvant GKRS for MTLE. Shown are axial sections from post-contrast T1-weighted MRIs for case #1 (A-C), case #2 (E-G), and case #3 (I-K). An axial section of a T2-weighted gradient recalled echo image supports the radiological diagnosis of cavernous malformation (indicated by arrowheads) for case #3 (L). Histopathological findings for case #1 (D) and case #2 (H) are shown using Hematoxylin-Eosin staining (scale bar=100 μm). RICM : radiation-induced cavernous malformations, GKRS : Gamma Knife radiosurgery, MTLE : mesial temporal lobe epilepsy, MRI : magnetic resonance imaging. 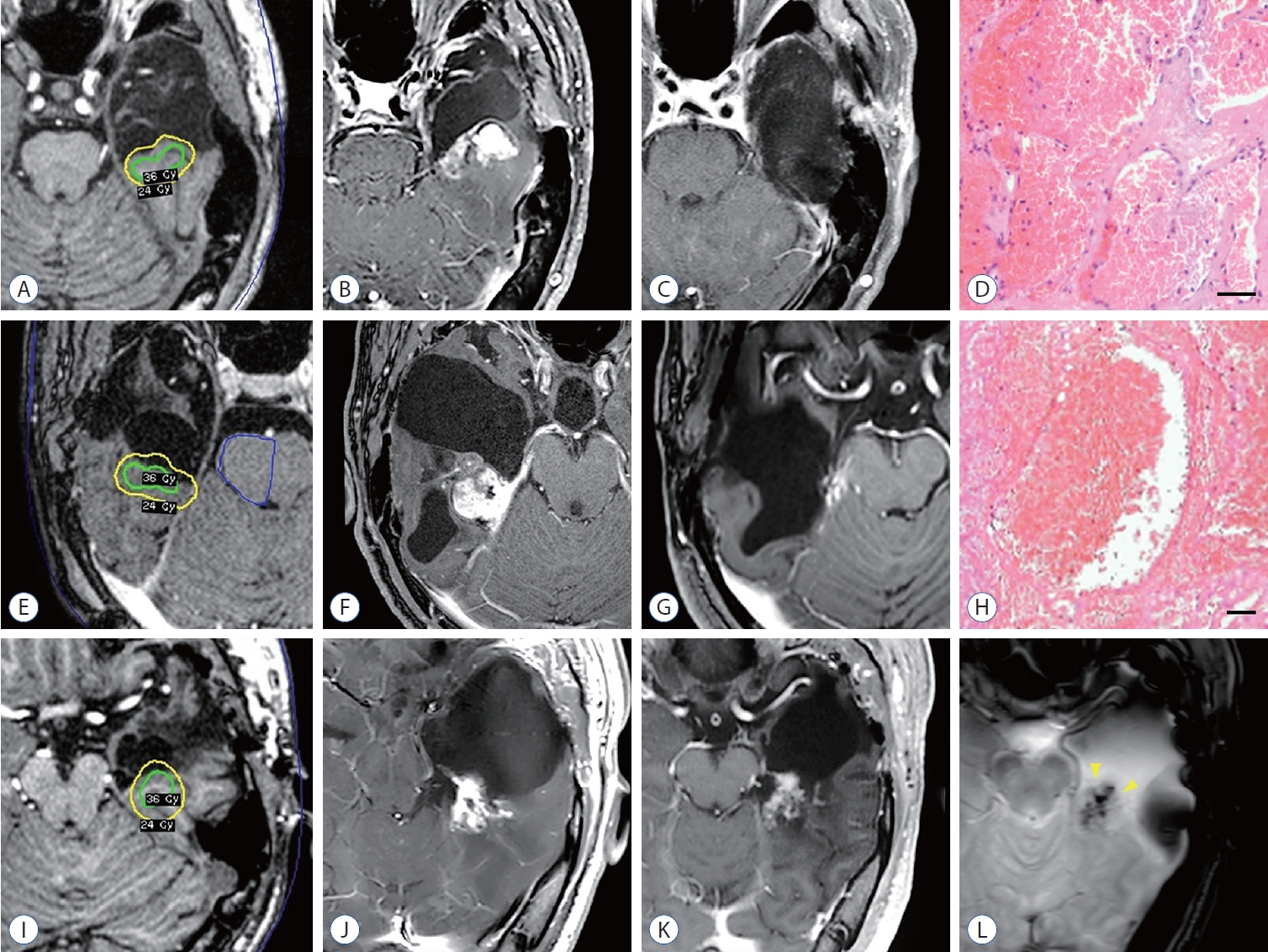
Table 1.
Patient demographics and radiosurgical outcomes
|
Study population (n=20) |
|
Age (years) |
|
|
At radiosurgery |
25.5 (31.0-42.8) |
|
At seizure onset |
11.5 (9.8-23.3) |
|
Sex, male |
14 (70.0) |
|
Disease duration* (years) |
12.0 (8.8-17.3) |
|
Time interval from ATL to GKRS† (years) |
5.5 (2.4-12.4) |
|
Structural etiology‡
|
|
|
HS |
13 (65.0) |
|
HS + CD |
3 (15.0) |
|
CD |
1 (5.0) |
|
Unspecified |
3 (15.0) |
|
Laterality |
|
|
Left |
10 (50.0) |
|
Seizure type |
|
|
CPS |
10 (50.0) |
|
CPS/G |
10 (50.0) |
|
Seizure frequency before GKRS |
|
|
<3 seizure days per year |
4 (20.0) |
|
3-6 seizure days per year |
5 (25.0) |
|
6-12 seizure days per year |
5 (25.0) |
|
≥1 seizure days per month |
6 (30.0) |
|
Treatment indication |
|
|
Primary GKRS |
2 (10.0) |
|
Adjuvant GKRS after ATL |
18 (90.0) |
|
Engel III |
15 (75.0) |
|
Engel IV |
3 (15.0) |
|
Target region |
|
|
Hippocampus & amygdala |
7 (35.0) |
|
Parahippocampal gyrus |
3 (15.0) |
|
Fusiform gyrus |
10 (50.0) |
|
Number of isocenters |
7 (4-11) |
|
Dose coverage (cm3) |
|
|
≥24 Gy (prescribed 50% isodose) |
4.6 (3.4-6.3) |
|
≥30 Gy |
2.6 (1.8-3.3) |
|
≥36 Gy |
1.0 (0.7-1.3) |
|
Maximum dose (Gy) |
48.2 (48.1-48.9) |
|
OAR constraint, brainstem (Gy) |
10.3 (8.0-11.9) |
|
Gradient index |
2.84 (2.73-2.98) |
|
Seizure control after GKRS§
|
|
|
Favorable |
14 (70.0) |
|
Early response, within 4 years |
7 (35.0) |
|
Late response, beyond 4 years |
7 (35.0) |
|
Unfavorable |
6 (30.0) |
|
ASM adjustments after GKRS |
|
|
Reduction |
8 (40.0) |
|
No reduction |
12 (60.0) |
References
2. Barbaro NM, Quigg M, Broshek DK, Ward MM, Lamborn KR, Laxer KD, et al : A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol 65 : 167-175, 2009   3. Barbaro NM, Quigg M, Ward MM, Chang EF, Broshek DK, Langfitt JT, et al : Radiosurgery versus open surgery for mesial temporal lobe epilepsy: the randomized, controlled ROSE trial. Epilepsia 59 : 1198-1207, 2018    4. Bartolomei F, Hayashi M, Tamura M, Rey M, Fischer C, Chauvel P, et al : Long-term efficacy of gamma knife radiosurgery in mesial temporal lobe epilepsy. Neurology 70 : 1658-1663, 2008   5. Chen N, Du SQ, Yan N, Liu C, Zhang JG, Ge Y, et al : Delayed complications after gamma knife surgery for intractable epilepsy. J Clin Neurosci 21 : 1525-1528, 2014   6. Cmelak AJ, Abou-Khalil B, Konrad PE, Duggan D, Maciunas RJ : Low-dose stereotactic radiosurgery is inadequate for medically intractable mesial temporal lobe epilepsy: a case report. Seizure 10 : 442-446, 2001   7. Cutsforth-Gregory JK, Lanzino G, Link MJ, Brown RD Jr, Flemming KD : Characterization of radiation-induced cavernous malformations and comparison with a nonradiation cavernous malformation cohort. J Neurosurg 122 : 1214-1222, 2015   8. Gross BA, Lin N, Du R, Day AL : The natural history of intracranial cavernous malformations. Neurosurg Focus 30 : E24, 2011   9. Kang JY, Wu C, Tracy J, Lorenzo M, Evans J, Nei M, et al : Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia 57 : 325-334, 2016    10. Kawai K, Suzuki I, Kurita H, Shin M, Arai N, Kirino T : Failure of low-dose radiosurgery to control temporal lobe epilepsy. J Neurosurg 95 : 883-887, 2001   11. Kawamura T, Onishi H, Kohda Y, Hirose G : Serious adverse effects of gamma knife radiosurgery for mesial temporal lobe epilepsy. Neurol Med Chir (Tokyo) 52 : 892-898, 2012   12. Kim MJ, Chang KW, Park SH, Chang WS, Chang JH, Chang JW, et al : Predictive factors of radiation-induced changes following single-session gamma knife radiosurgery for arteriovenous malformations. J Clin Med 10 : 2186, 2021    13. Koike T, Yanagimachi N, Ishiguro H, Yabe H, Yabe M, Morimoto T, et al : High incidence of radiation-induced cavernous hemangioma in longterm survivors who underwent hematopoietic stem cell transplantation with radiation therapy during childhood or adolescence. Biol Blood Marrow Transplant 18 : 1090-1098, 2012   14. Lee EM, Kang JK, Kim SJ, Hong SH, Ko TS, Lee SA, et al : Gamma knife radiosurgery for recurrent or residual seizures after anterior temporal lobectomy in mesial temporal lobe epilepsy patients with hippocampal sclerosis: long-term follow-up results of more than 4 years. J Neurosurg 123 : 1375-1382, 2015   15. Lew SM, Morgan JN, Psaty E, Lefton DR, Allen JC, Abbott R : Cumulative incidence of radiation-induced cavernomas in long-term survivors of medulloblastoma. J Neurosurg 104( 2 Suppl):103-107, 2006   16. McGonigal A, Sahgal A, De Salles A, Hayashi M, Levivier M, Ma L, et al : Radiosurgery for epilepsy: systematic review and International Stereotactic Radiosurgery Society (ISRS) practice guideline. Epilepsy Res 137 : 123-131, 2017   17. Nimjee SM, Powers CJ, Bulsara KR : Review of the literature on de novo formation of cavernous malformations of the central nervous system after radiation therapy. Neurosurg Focus 21 : e4, 2006  19. Régis J, Rey M, Bartolomei F, Vladyka V, Liscak R, Schröttner O, et al : Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia 45 : 504-515, 2004   20. Srikijvilaikul T, Najm I, Foldvary-Schaefer N, Lineweaver T, Suh JH, Bingaman WE : Failure of gamma knife radiosurgery for mesial temporal lobe epilepsy: report of five cases. Neurosurgery 54 : 1395-1402; discussion 1402-1404, 2004   21. Trifiletti DM, Redmond KJ, Kim MM, Soltys SG, Milano MT, HattangadiGluth JA : Novel applications of stereotactic radiosurgery beyond oncology: prospective trials in functional radiosurgery. Int J Radiat Oncol Biol Phys 115 : 4-6, 2023   23. Vojtech Z, Vladyka V, Kalina M, Nespor E, Seltenreichová K, Semnická J, et al : The use of radiosurgery for the treatment of mesial temporal lobe epilepsy and long-term results. Epilepsia 50 : 2061-2071, 2009   24. Winkler EA, Rutledge C, Ward M, Tihan T, Sneed PK, Barbaro N, et al : Radiation-induced cavernous malformation as a late sequelae of stereotactic radiosurgery for epilepsy. Cureus 10 : e2308, 2018   
|
|


















